

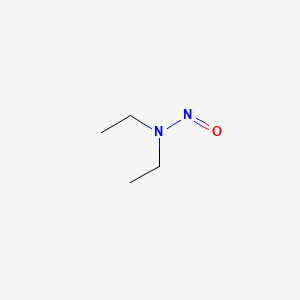

1. Diethylnitrosamine
2. N Nitrosodiethylamine
3. Nitrosodiethylamine
1. 55-18-5
2. Diethylnitrosamine
3. Diethylnitrosoamine
4. Ndea
5. N-ethyl-n-nitrosoethanamine
6. N,n-diethylnitrous Amide
7. Nitrosodiethylamine
8. N,n-diethylnitrosamine
9. N,n-diethylnitrosoamine
10. Ethanamine, N-ethyl-n-nitroso-
11. Diethylamine, N-nitroso-
12. Diethylnitrosamide
13. Dena
14. Diaethylnitrosamin
15. N-nitroso-n,n-diethylamine
16. Den (mutagen)
17. Ethylamine, N-nitrosodi-
18. Den
19. Rcra Waste Number U174
20. Nsc 132
21. N-nitroso-diaethylamine
22. 3iq78ttx1a
23. 1,1-diethyl-2-oxohydrazine
24. Chebi:34873
25. Nsc-132
26. N-nitrosodiethlamine
27. Mfcd00013890
28. N-diethylnitrosamine
29. Dsstox_cid_1028
30. Dsstox_rid_75913
31. Dsstox_gsid_21028
32. N-nitrosodiethyl-d10-amine
33. Nitrosamine, Diethyl-
34. Diaethylnitrosamin [german]
35. Cas-55-18-5
36. N-ethyl-n-nitroso-ethanamine
37. Ccris 239
38. Hsdb 4001
39. N-nitroso-diaethylamine [german]
40. Einecs 200-226-1
41. Rcra Waste No. U174
42. Unii-3iq78ttx1a
43. Brn 1744991
44. N-nitrosodiethylamine (ndea)
45. Ai3-62031
46. Diethylnitrosamine
47. Diethyl-nitroso-amine
48. N-nitroso-diethylamine
49. Diaethylnitrosamin;ndma
50. N-ethyl-n-nitroso-ethylamine
51. N-nitrosodiethylamine, Liquid
52. Mls002415760
53. Bidd:er0527
54. Schembl106629
55. Wln: Onn2&2
56. Chembl164290
57. Nsc132
58. 1,1-diethyl-2-oxohydrazine #
59. Dtxsid2021028
60. Hms3039g08
61. N-nitrosodiethylamine [mi]
62. N-nitrosodiethylamine, Isopac(r)
63. Bcp19136
64. Hy-n7434
65. Zinc3875370
66. N-nitrosodiethylamine [hsdb]
67. N-nitrosodiethylamine [iarc]
68. Tox21_201255
69. Tox21_300533
70. Stl220642
71. Akos015903105
72. N-nitrosodiethylamine [usp-rs]
73. N-nitrosodiethylamine, >=99.0% (gc)
74. Ncgc00091350-01
75. Ncgc00091350-02
76. Ncgc00091350-03
77. Ncgc00091350-04
78. Ncgc00254309-01
79. Ncgc00258807-01
80. Bs-17234
81. N-nitrosodiethylamine, Analytical Standard
82. Smr001370919
83. Sy053647
84. Db-052695
85. Cs-0128799
86. D0516
87. Ft-0631240
88. N-nitrosodiethylamine 0.1 Mg/ml In Methanol
89. F14922
90. N-nitroso-diethylamine 100 Microg/ml In Methanol
91. N-nitroso-diethylamine 1000 Microg/ml In Methanol
92. Q22138388
93. N-nitroso-diethylamine 1000 Microg/ml In Dichloromethane
| Molecular Weight | 102.14 g/mol |
|---|---|
| Molecular Formula | C4H10N2O |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 102.079312947 g/mol |
| Monoisotopic Mass | 102.079312947 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 51.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
/MILK/ In goats, 1 hr after oral administration of 30 mg/kg bw nitrosodiethylamine, there were 11.4 mg/kg nitrosodiethylamine in milk and 11.9 mg/kg in blood. Only traces were found in milk and none in blood after 24 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V17 104 (1978)
Autoradiographic studies indicated that non-metabolized N-nitrosodiethylamine passed to fetuses with even distribution in most fetal tissues on all studied days of gestation (day 12, 14, 16, 16 and 18) in mice. Results also indicated metabolism of the substance in mucosa of fetal bronchial tree and liver on day 18 of gestation.
Brittebo EB et al; Acta Pharmacacol Toxicol 48 (4): 355-63 (1981)
Inhibition of sulfotransferase by 2,6-dichloro-4-nitrophenol completely abolished the genotoxic potential of N-nitrosodiethanolamine in rat liver as indicated by the induction of DNA single-strand breaks. The DNA strand-breaking potential of N-nitroso-2-hydroxymorpholine, a metabolite of N-nitrosodiethanolamine formed by alcohol dehydrogenase -mediated oxidation, was also almost quantitatively abolished. In contrast to these beta-hydroxylated nitrosamines, the effectiveness of N-nitrosodiethylamine remained unaffected by 2,6-dichloro-4-nitrophenol with respect to its DNA damaging potential. ... A new activation mechanism for N-nitrosodiethanolamine is proposed: N-nitrosodiethanolamine is transformed at first by alcohol dehydrogenase into the cyclic hemiacetal N-nitroso-2-hydroxymorpholine. This cyclic beta-hydroxynitrosamine appears to be a substrate for sulfotransferase. The resulting sulfate conjugate is suggested to be ultimate genotoxic electrophile. However, the results do not exclude the possibility that N-nitrosodiethanolamine itself undergoes sulfate conjugation.
PMID:3456349 Sterzel W, Eisenbrand G; J Cancer Res Clin Oncol 111 (1): 20-4 (1986)
Oxidative N-deethylation of NDEA accounts for the production of CO2 and alkylating species in vivo. The rate of metabolism of NDEA by slices of organs from rats and hamsters in vitro has been measured, and a correlation made between the degree of metabolism and the distribution of induced tumors. After administration of NDEA to rats or hamsters, several ethylated derivatives were produced in liver and kidney nucleic acids. These included 7-ethylguanine, O6-ethylguanine and 3-ethyladenine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V17 105 (1978)
... Evidence suggests that nitrosodiethylamine requires metabolic activation in order to exert its carcinogenic and toxic effects. ... N-nitrosoethyl-N-(2-hydroxyethyl)amine and N-nitrosoethyl-N-(carboxymethyl)amine have been detected in urine of rats. ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V17 105 (1978)
Possible relationships between structure and metabolism of nitrosamines have been investigated in the rat small intestine. Isolated segments of jejunum and ileum were perfused from the luminal side for 2 hr with a Tyrode solution containing one of four symmetrical dialkylnitrosamines with 2-5 carbon atoms per side chain, all (14)C-labeled at the alpha position, or one of two unsymmetrical nitrosamines, N-nitroso-tert-butylmethylamine and N-nitrosomethylbenzylamine, (14)C-labeled in the methyl group. Besides measurement of (14)C to intestinal tissue, the absorbed fluid (absorbate) as well as the perfusion medium and tissue homogenates were analyzed by for the presence of polar metabolites to assess the intestinal metabolism of nitrosamines. Neither N-nitrosodiethylamine nor the two unsymmetrical nitrosamines were metabolized to any significant extent.
PMID:3087650 Richter E et al; Carcinogenesis 7 (7): 1207-14 (1986)
Nitrosodiethylamine has known human metabolites that include N-Nitrosoethanamine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... It is shown that the two nitrosamines N-nitrosodiethylamine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bind to nicotinic cholinergic receptors in hamster lung. Binding of the nitrosamines as well as nicotine to this receptor stimulates proliferation of human lung carcinoid cells in vitro. These data suggest chronic stimulation of nicotinic receptors by nicotine and nitrosamines in smokers as one of the molecular events responsible for stimulation of neuroendocrine cell proliferation and ultimately the development of lung tumors with neuroendocrine differentiation. ...
Schuller HM; Cancer Res 52 (9 Suppl): 2723s-6s (1992)
