
1. Arolac
2. Carbamide, Methylergol
3. Cuvalit
4. Dopergin
5. Dopergine
6. Hydrochloride, Lisuride
7. Hydrogen Maleate, Lysuride
8. Lisuride
9. Lisuride Hydrochloride
10. Lisuride Maleate (1:1)
11. Lisuride Maleate, (8beta)-isomer
12. Lisuride Mesylate
13. Lisuride Phosphate (1:1)
14. Lisuride, (8alpha)-(+-)-isomer
15. Lysenyl
16. Lysurid
17. Lysuride Hydrogen Maleate
18. Maleate, Lisuride
19. Mesylate, Lisuride
20. Methylergol Carbamide
21. Revanil
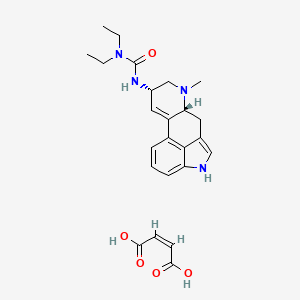
1. Lisuride Hydrogen Maleate
2. 19875-60-6
3. Lisuride Maleate [jan]
4. Lysenyl Hydrogen Maleate
5. Uv1635n8xw
6. R(+)-lisuride Hydrogen Maleate
7. Lisuride (maleate)
8. Lysuride Maleate
9. Cuvalit
10. Lysenyl
11. Lisuride Maleate (jan)
12. Lysenyl Bimaleate
13. 3-(9,10-didehydro-6-methylergolin-8-alpha-yl)-1,1-diethylurea Maleate (1:1)
14. 3-(9,10-didehydro-6-methylergolin-8-yl)-1,1-diethylurea Hydrogen Maleate
15. Urea, N'-((8-alpha)-9,10-didehydro-6-methylergolin-8-yl)-n,n-diethyl-, (z)-2-butenedioate
16. Chebi:31776
17. Urea, N'-[(8alpha)-9,10-didehydro-6-methylergolin-8-yl]-n,n-diethyl-, (2z)-2-butenedioate (1:1)
18. Einecs 243-387-3
19. Unii-uv1635n8xw
20. 1-((5r,8s)-6-methyl-9,10-didehydro-8-ergolinyl)-3,3-diethylurea Hydrogen Maleate
21. Lisuride Maleate [mi]
22. Schembl364392
23. Chembl1257128
24. Lisuride Maleate [mart.]
25. Lisuride Maleate [who-dd]
26. Dtxsid501017378
27. Akos025142010
28. Urea, 3-(9,10-didehydro-6-methylergolin-8-alpha-yl)-1,1-diethyl-, Maleate (1:1)
29. Hy-110080
30. Cs-0032924
31. D01462
32. Sr-01000075387
33. Sr-01000075387-1
34. Q27114683
35. N'-[(8?)-9,10-didehydro-6-methylergolin-8-yl]-n,n-diethylurea Maleate
36. 3-(9,10-didehydro-6-methylergolin-8.alpha.-yl)-1,1-diethylurea Maleate (1:1)
37. R(+)-n'-[(8alpha)-9,10-didehydro-6-methylergolin-8-yl]-n,n,-diethylurea Hydrogen Maleate
| Molecular Weight | 454.5 g/mol |
|---|---|
| Molecular Formula | C24H30N4O5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 454.22162007 g/mol |
| Monoisotopic Mass | 454.22162007 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 663 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
