


1. Choline Glycerophospholipids
2. Choline Phosphoglycerides
3. Choline, Phosphatidyl
4. Cholines, Phosphatidyl
5. Glycerophospholipids, Choline
6. Phosphatidyl Choline
7. Phosphatidyl Cholines
8. Phosphatidylcholine
9. Phosphatidylcholines
10. Phosphoglycerides, Choline
1. L-alpha-lecithin
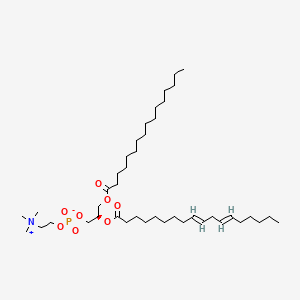
2. Pc(16:0/18:2(9e,12e))
3. Lmgp01010592
4. 1-hexadecanoyl-2-(9e,12e-octadecadienoyl)-sn-glycero-3-phosphocholine
5. 2-linoleoyl-1-palmitoyl-sn-glycero-3-phosphocholine
6. Pc(16:0/18:2)
7. Phosphatidylcholines
8. 3,5,8-trioxa-4-phosphahexacosa-17,20-dien-1-aminium, 4-hydroxy-n,n,n-trimethyl-9-oxo-7-[[(1-oxohexadecyl)oxy]methyl]-, Inner Salt, 4-oxide, (r)-
9. Phospholutein
10. 8002-43-5
11. L-a-phosphatidylcholine
12. Phosphatidylcholines Soya
13. Soybean Phosphatidylcholine
14. Bmse001027
15. L-alpha-phosphatidylcholine Solution
16. 97281-47-5
17. J-011253
18. 2-linoleoyl-1-palmitoyl-sn-glyc-ero-3-phosphocholine
19. 1-hexadecanoyl-2-(cis-9,12-octadecadienoyl)-sn-glycero-3-phosphocholine
| Molecular Weight | 758.1 g/mol |
|---|---|
| Molecular Formula | C42H80NO8P |
| XLogP3 | 12.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 40 |
| Exact Mass | 757.56215551 g/mol |
| Monoisotopic Mass | 757.56215551 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 941 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Surface-Active Agents
National Library of Medicine's Medical Subject Headings. Lecithins. Online file (MeSH, 2016). Available from, as of June 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/EXPL THER/ Endosulfan is an organochlorine pesticide commonly found in aquatic environments that has been found to reduce thermal tolerance of fish. Lipotropes such as the food additive, Lecithin has been shown to improve thermal tolerance in fish species. This study was conducted to evaluate the role of lipotropes (lecithin) for enhancing the thermal tolerance of Chanos chanos reared under sublethal low dose endosulfan-induced stress. Two hundred and twenty-five fish were distributed randomly into five treatments, each with three replicates. Four isocaloric and isonitrogenous diets were prepared with graded levels of lecithin: normal water and fed with control diet (En0/L0), endosulfan-treated water and fed with control diet (En/L0), endosulfan-treated water and fed with 1% (En/L1%), 1.5% (En/L 1.5%) and 2% (En/L 2%) lecithin supplemented feed. The endosulfan in treated water was maintained at the level of 1/40th of LC50 (0.52ppb). At the end of the five weeks, critical temperature maxima (CTmax), lethal temperature maxima (LTmax), critical temperature minima (CTmin) and lethal temperature minima (LTmin) were Determined. There was a significant (P<0.01) effect of dietary lecithin on temperature tolerance (CTmax, LTmax, CTmin and LTmin) of the groups fed with 1, 1.5 and 2% lecithin-supplemented diet compared to control and endosulfan-exposed groups. Positive correlations were observed between CT max and LTmax (R(2)=0.934) as well as between CTmin and LTmin (R(2)=0.9313). At the end of the thermal tolerance study, endosulfan-induced changes in cellular stress enzymes (Catalase, SOD and GST in liver and gill and neurotansmitter enzyme, brain AChE) were significantly (p<0.01) improved by dietary lecithin. We herein report the role of lecithin in enhancing the thermal tolerance and protection against cellular stress in fish exposed to an organochlorine pesticide.
PMID:25455939 Kumar N et al; J Therm Biol 46: 40-6 (2014)
/EXPL THER/ Suitability of liquid lecithin (i.e., solution of lecithin in soy bean oil with ~60% w/w of phospholipids) for formation of gels, upon addition of water solution of poloxamer 407, was investigated, and formulated systems were evaluated as carriers for percutaneous delivery of ibuprofen. Formulation study of pseudo-ternary system liquid lecithin/poloxamer 407/water at constant liquid lecithin/poloxamer 407 mass ratio (2.0) revealed that minimum concentrations of liquid lecithin and poloxamer 407 required for formation of gel like systems were 15.75% w/w and 13.13% w/w, respectively, while the maximum content of water was 60.62% w/w. The systems comprising water concentrations in a range from 55 to 60.62% w/w were soft semisolids suitable for topical application, and they were selected for physicochemical and biopharmaceutical evaluation. Analysis of conductivity results and light microscopy examination revealed that investigated systems were water dilutable dispersions of spherical oligolamellar associates of phospholipids and triglyceride molecules in the copolymer water solution. Rheological behavior evaluation results indicated that the investigated gels were thermosensitive shear thinning systems. Ibuprofen (5% w/w) was incorporated by dispersing into the previously prepared carriers. Drug-loaded systems were physically stable at storage temperature from 5 +/- 3 C to 40 +/- 2 C, for 30 days. In vitro ibuprofen release was in accordance with the Higuchi model (rH>0.95) and sustained for 12 hr. The obtained results implicated that formulated LLPBGs, optimized regarding drug release and organoleptic properties, represent promising carriers for sustained percutaneous drug delivery of poorly soluble drugs.
PMID:26002567 Djekic L et al; Int J Pharm 490 (1-2): 180-9 (2015)
/EXPL THER/ Some dietary factors could inhibit lead toxicity. The aim of this study was to evaluate the effect of dietary compounds rich in unsaturated fatty acids (FA) on blood lead level, lipid metabolism, and vascular reactivity in rats. Serum metallothionein and organs' lead level were evaluated with the aim of assessing the possible mechanism of unsaturated FA impact on blood lead level. For three months, male Wistar rats that were receiving drinking water with (100 ppm Pb) or without lead acetate were supplemented per os daily with virgin olive oil or linseed oil (0.2 mL/kg b.w.) or egg derived lecithin fraction: "super lecithin" (50 g/kg b.w.). Mesenteric artery was stimulated ex vivo by norepinephrine (NE) administered at six different doses. Lecithin supplementation slightly reduced pressor responses of artery to NE. Lead administered to rats attenuated the beneficial effect of unsaturated FA on lipid metabolism and vascular reactivity to adrenergic stimulation. On the other hand, the super lecithin and linseed oil that were characterized by low omega-6 to omega-3 ratio (about 1) reduced the blood lead concentration. This effect was observed in lead poisoned rats (p < 0.0001) and also in rats nonpoisoned with lead (p < 0.05).
PMID:26075218 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4446462 Skoczynska A et al; Biomed Res Int 2015:189190 doi: 10.1155/2015/189190 (2015) Epub 2015 May 14
For more Therapeutic Uses (Complete) data for LECITHINS (9 total), please visit the HSDB record page.
