

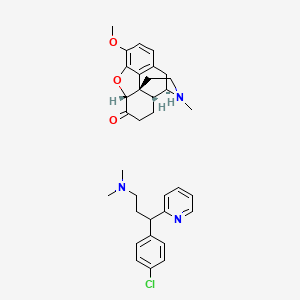

1. Chlorpheniramine Mixture With Hydrocodone
2. 131219-97-1
3. Vituz
4. Tuzistra Xr
5. Tussionex Pennkinetic
6. S-t Forte 2
7. Tussicaps Extended-release
8. Tussionex
9. Chlorpheniramine / Hydrocodone
10. Chlorpheniramine Polistirex / Hydrocodone Polistirex
11. Chlorpheniramine Polistirex And Hydrocodone Polistirex
12. Hydrocodone Polistirex And Chlorpheniramine Polistirex
13. Schembl2522788
14. Dtxsid50156960
15. Hydrocodone Conbination Product 15 Mg/du
| Molecular Weight | 574.1 g/mol |
|---|---|
| Molecular Formula | C34H40ClN3O3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 573.2758198 g/mol |
| Monoisotopic Mass | 573.2758198 g/mol |
| Topological Polar Surface Area | 54.9 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 758 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Hydrocodone polistirex and chlorpheniramine polistirex |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate/5ml; eq 8mg maleate/5ml |
| Market Status | Prescription |
| Company | Tris Pharma |
| 2 of 6 | |
|---|---|
| Drug Name | Tussicaps |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate; eq 5mg bitartrate; eq 4mg maleate; eq 8mg maleate |
| Market Status | Prescription |
| Company | Ecr Pharma |
| 3 of 6 | |
|---|---|
| Drug Name | Tussionex pennkinetic |
| PubMed Health | Hydrocodone and Chlorpheniramine Polistirex (Oral route) |
| Drug Classes | Antitussive, Opioid/Antihistamine Combination, Chlorpheniramine |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate/5ml; eq 8mg maleate/5ml |
| Market Status | Prescription |
| Company | Ucb |
| 4 of 6 | |
|---|---|
| Drug Name | Hydrocodone polistirex and chlorpheniramine polistirex |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate/5ml; eq 8mg maleate/5ml |
| Market Status | Prescription |
| Company | Tris Pharma |
| 5 of 6 | |
|---|---|
| Drug Name | Tussicaps |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate; eq 5mg bitartrate; eq 4mg maleate; eq 8mg maleate |
| Market Status | Prescription |
| Company | Ecr Pharma |
| 6 of 6 | |
|---|---|
| Drug Name | Tussionex pennkinetic |
| PubMed Health | Hydrocodone and Chlorpheniramine Polistirex (Oral route) |
| Drug Classes | Antitussive, Opioid/Antihistamine Combination, Chlorpheniramine |
| Active Ingredient | Chlorpheniramine polistirex; hydrocodone polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 10mg bitartrate/5ml; eq 8mg maleate/5ml |
| Market Status | Prescription |
| Company | Ucb |
