


1. 6 Alpha-fluoroclobetasol 17-propionate
2. 6-fluoroclobetasol 17-propionate
3. Cgp 14 458
4. Cgp 14458
5. Cgp-14458
6. Halobetasol
7. Ulobetasol
8. Ultravate
1. Ulobetasol Propionate
2. 66852-54-8
3. Ultravate
4. Miracorten
5. Halobetasol (propionate)
6. Bmy-30056
7. Jemdel
8. Cgp-14458
9. Cgp-14,458
10. 91a0k1ty3z
11. Unii-91a0k1ty3z
12. Halobetasol Propionate [usan]
13. Ultravate (tn)
14. Ncgc00167451-01
15. Halobetasol Propionate [usan:usp]
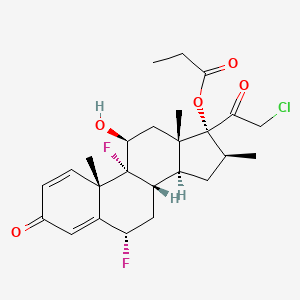
16. [(6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-(2-chloroacetyl)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Propanoate
17. Bmy 30056
18. Bryhali
19. Halobetasol-propionate
20. Bmy-30056;cgp-14458;ulobetasol Propionate
21. Dsstox_cid_26636
22. Dsstox_rid_81782
23. Dsstox_gsid_46636
24. Schembl33858
25. Halobetasol Propionate (usp)
26. Chembl1200908
27. Dtxsid6046636
28. Chebi:135782
29. Halobetasol Propionate [mi]
30. (6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-(2-chloroacetyl)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl Propionate
31. Hy-b0878
32. Zinc3920028
33. Tox21_112455
34. Mfcd00866013
35. S4089
36. Halobetasol Propionate [vandf]
37. Ulobetasol Propionate [mart.]
38. Akos025310143
39. Ulobetasol Propionate [who-dd]
40. Ccg-269585
41. Cs-4305
42. Halobetasol Propionate [usp-rs]
43. Ncgc00373230-02
44. 21-chloro-6alpha,9-difluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17-propionate
45. Cas-66852-54-8
46. Halobetasol Propionate [orange Book]
47. Halobetasol Propionate [usp Monograph]
48. D04409
49. Duobrii Component Halobetasol Propionate
50. Ab01566925_01
51. Idp-118 Component Halobetasol Propionate
52. 852h548
53. Halobetasol Propionate Component Of Duobrii
54. Sr-01000942239
55. Sr-01000942239-1
56. (6alpha,11beta,16beta)-21-chloro-6,9-difluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)pregna-1,4-diene-3,20-dione
57. 21-chloro-6.alpha.,9-difluoro-11.beta.,17-dihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17-propionate
58. Pregna-1,4-diene-3,20-dione, 21-chloro-6,9-difluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-, (6.alpha.,11.beta.,16.beta.)-
59. Pregna-1,4-diene-3,20-dione, 21-chloro-6,9-difluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-, (6alpha,11beta,16beta)-
| Molecular Weight | 485.0 g/mol |
|---|---|
| Molecular Formula | C25H31ClF2O5 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 484.1828081 g/mol |
| Monoisotopic Mass | 484.1828081 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 964 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Halobetasol propionate |
| Drug Label | Halonate Halobetasol Propionate Ointment, 0.05% contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory... |
| Active Ingredient | Halobetasol propionate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Taro; Fougera Pharms; Perrigo; G And W Labs; Perrigo Israel |
| 2 of 2 | |
|---|---|
| Drug Name | Halobetasol propionate |
| Drug Label | Halonate Halobetasol Propionate Ointment, 0.05% contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory... |
| Active Ingredient | Halobetasol propionate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Taro; Fougera Pharms; Perrigo; G And W Labs; Perrigo Israel |
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
