


1. Bms 284756
2. Bms-284756
3. Bms284756
1. 194804-75-6
2. Ganefloxacin
3. T-3811
4. T 3811
5. Bms284756
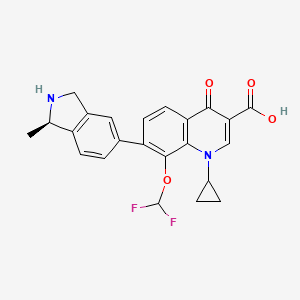
6. (r)-1-cyclopropyl-8-(difluoromethoxy)-7-(1-methylisoindolin-5-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
7. 1-cyclopropyl-8-(difluoromethoxy)-7-[(1r)-1-methyl-2,3-dihydro-1h-isoindol-5-yl]-4-oxoquinoline-3-carboxylic Acid
8. V72h9867wb
9. Bms-284756
10. Garenfloxacin
11. 1-cyclopropyl-8-(difluoromethoxy)-7-[(1r)-1-methyl-2,3-dihydro-1h-isoindol-5-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
12. 194804-75-6 Pound Not223652-90-2
13. Ncgc00181770-01
14. Garenoxacin [inn:ban]
15. Bms 284756 (*mesylate Salt*)
16. Garenoxacine
17. Garenoxacino
18. Garenoxacinum
19. Unii-v72h9867wb
20. T-3811mea
21. Bms-284756 (*mesylate Salt*)
22. Garenoxacin [mi]
23. Garenoxacin [inn]
24. Garenoxacin [mart.]
25. Garenoxacin [who-dd]
26. Chembl215303
27. Schembl2103727
28. Amy8881
29. Dtxsid30173135
30. Chebi:131716
31. Bcpp000224
32. Bcp01415
33. Ex-a3040
34. Zinc3585048
35. Bdbm50481219
36. S5908
37. Akos015920281
38. Bcp9000709
39. Cs-4989
40. Db06160
41. 1-cyclopropyl-8-(difluoromethoxy)-7-[(1r)-(1-methyl-2,3-dihydro-1h-5-isoindolyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic Acid
42. 1-cyclopropyl-8-(difluoromethoxy)-7-[(1r)-1-methylisoindolin-5-yl]-4-oxo-quinoline-3-carboxylic Acid
43. 1-cyclopropyl-8-(difluoromethoxy)-7-[(1r)-2,3-dihydro-1-methyl-1h-isoindol-5-yl]-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid
44. Hy-17460
45. A16856
46. 804g756
47. J-012614
48. J-521409
49. Q3758306
50. (r)-1-cyclopropyl-8-difluoromethoxy-7-(1-methyl-2, 3-dihydro-1h-5-isoindolyl)-4-oxo-1, 4-dihydro-3-quinolinecarboxylic Acid
51. (r)-1-cyclopropyl-8-difluoromethoxy-7-(1-methyl-2,3-dihydro-1h-5-isoindolyl)-4-oxo-1,4-dihydro-3-quinoline-carboxylic Acid
52. (r)-1-cyclopropyl-8-difluoromethoxy-7-(1-methyl-2,3-dihydro-1h-5-isoindolyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic Acid
| Molecular Weight | 426.4 g/mol |
|---|---|
| Molecular Formula | C23H20F2N2O4 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 426.13911345 g/mol |
| Monoisotopic Mass | 426.13911345 g/mol |
| Topological Polar Surface Area | 78.9 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 771 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in bacterial infection.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA19 - Garenoxacin
