


1. Caproate, Fluocortolone
2. Fluocortolone
3. Fluocortolone Pivalate
4. Pivalate, Fluocortolone
5. Ultralan
1. 303-40-2
2. Fluocortolone 21-hexanoate
3. Fluocortolone Hexanoate
4. Sh 770
5. Fluocortolone Caproate [usan]
6. Einecs 206-140-0
7. Unii-90893p8662
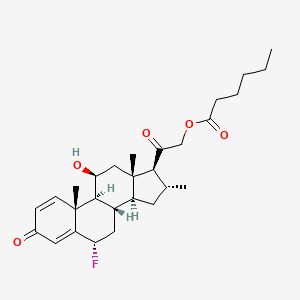
8. 6alpha-fluoro-11beta,21-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 21-hexanoate
9. [2-[(6s,8s,9s,10r,11s,13s,14s,16r,17s)-6-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Hexanoate
10. Fluocortolone Caproate (usan)
11. 90893p8662
12. Ficoid
13. Fluocoutolone Hexanoate
14. Ultralanum
15. F1uocorto1one Caproate
16. Schembl2107766
17. Chembl2107415
18. C28h39fo5
19. Dtxsid30184387
20. Fluocortolone 21-caproate
21. Chebi:177866
22. C28-h39-f-o5
23. Sh-770
24. Fluocortolone Caproate [mart.]
25. Fluocortolone Caproate [who-dd]
26. Fluocortolone 21-hexanoate [mi]
27. D04219
28. Q27271302
29. [2-[(6s,8s,9s,10r,11s,13s,14s,16r,17s)-6-luoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Hexanoate
30. 6.alpha.-fluoro-11.beta.-hydroxy-21-hexanoyloxy-16.alpha.-methylpregna-1,4-diene-3,20-dione
31. Hexanoic Acid, 21-ester With 6.alpha.-fluoro-11.beta.,21-dihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione
32. Pregna-1,4-diene-3,20-dione, 6-fluoro-11,21-dihydroxy-16-methyl-21-((1-oxohexyl)oxy)-, (6alpha,11beta,16alpha)-
33. Pregna-1,4-diene-3,20-dione, 6-fluoro-11-hydroxy-16-methyl-21-((1-oxohexyl)oxy)-, (6.alpha.,11.beta.,16.alpha.)-
34. Pregna-1,4-diene-3,20-dione, 6.alpha.-fluoro-11.beta.,21-dihydroxy-16.alpha.-methyl-, 21-hexanoate
1. Caproate, Fluocortolone
2. Fluocortolon
3. Fluorcortolone
4. Fluorocortolone
5. Fluocortolone
6. Flucortolone
7. Ultralan
| Molecular Weight | 474.6 g/mol |
|---|---|
| Molecular Formula | C28H39FO5 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 80.7 |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 909 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
