
1. 104040-78-0
2. Shibagen
3. Flazasulfuron [iso]
4. Sl-160
5. Ok-1166
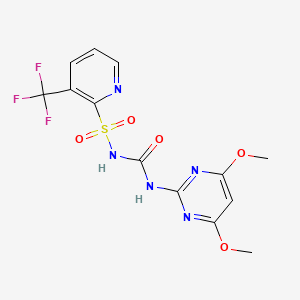
6. 1-(4,6-dimethoxypyrimidin-2-yl)-3-(3-trifluoromethyl-2-pyridylsulfonyl)urea
7. 1-(4,6-dimethoxypyrimidin-2-yl)-3-[3-(trifluoromethyl)pyridin-2-yl]sulfonylurea
8. N-(((4,6-dimethoxy-2-pyrimidinyl)amino)carbonyl)-3-(trifluoromethyl)-2-pyridinesulfonamide
9. 3sb13wwv30
10. Chebi:81749
11. 2-pyridinesulfonamide, N-(((4,6-dimethoxy-2-pyrimidinyl)amino)carbonyl)-3-(trifluoromethyl)-
12. Flazasulfuron 100 Microg/ml In Acetonitrile
13. Shibagen [japanese]
14. Unii-3sb13wwv30
15. Hsdb 7957
16. Katana
17. Flazasulfuron [mi]
18. N-(4,6-dimethoxy-2-pyrimidinylaminocarbonyl)-3-(trifluoromethyl)-2-pyridinesulfonamide
19. Schembl54678
20. Chembl1878071
21. Dtxsid3034610
22. Zinc6088311
23. Mfcd00274594
24. Akos015900343
25. Ncgc00164266-01
26. Db-040506
27. Cs-0440149
28. Ft-0642552
29. C18441
30. F21476
31. Flazasulfuron, Pestanal(r), Analytical Standard
32. 040f780
33. A800883
34. J-001097
35. Q3073504
36. 1-(4,6-dimethoxy-2-pyrimidinyl)-3-[[3-(trifluoromethyl)-2-pyridinyl]sulfonyl]urea
37. Flazasulfuron1-(4,6-dimethoxypyrimidin-2-yl)-3-(3-trifluoromethyl-2-pyridylsulfonyl)urea
38. N-((4,6-dimethoxypyrimidin-2-yl)carbamoyl)-3-(trifluoromethyl)pyridine-2-sulfonamide
39. N-(4,6-dimethoxypyrimidin-2-ylcarbamoyl)-3-(trifluoromethyl)pyridine-2-sulfonamide
40. Flazasulfuron;1-(4,6-dimethoxypyrimidin-2-yl)-3-(3-trifluoromethyl-2-pyridylsulfonyl)urea
| Molecular Weight | 407.33 g/mol |
|---|---|
| Molecular Formula | C13H12F3N5O5S |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 407.05112416 g/mol |
| Monoisotopic Mass | 407.05112416 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 602 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Rapidly and extensively absorbed(90%), widely distributed and almost completely excreted (up to 90%) within 7 days, mainly in urine.
MacBean C, ed. Flazasulfuron (104040-78-0). In: The e-Pesticide Manual, 15th Edition, Version 5.0.1 (2010). Surrey UK, British Crop Protection Council.
A series of studies were conducted to evaluate the absorption, distribution, metabolism, and excretion of (14)C-SL-160(P)/flazasulfuron/ in groups of 4 or 5 rats (CRL: CD BR VAF/Plus)/sex. In these studies, (14)C-SL-160(P) (CP-1385; 55.8 mCi/mMole; radiolabel purity variously reported as 97.29%, 97.5%. 98.49%., 99.2% and >98%) was administered orally by gavage at doses of 2 or 50 mg/kg or 2 mg/kg . In /one study/, rats had received daily oral gavage doses of nonlabeled SL-160 (Y-920205; 98.9% purity) for 14 days, prior to oral dosing with (14)C-SL-160(P) on Day 15. ... Differences were generally not noted between dose levels or between single versus repeated dose administration, but were noted between males and females. The studies demonstrated that 14C SL-160(P) is rapidly absorbed following a single oral dose of 2 or 50 mg/kg. By 48 hours post exposure, males and females absorbed 94% and 93% of the low dose, respectively, and 86% and 93% of the high dose, respectively. A similar pattern was observed when low- and high-dose animals were followed up to 168 hours with little difference noted between a single or repeated exposure. Elimination via the urine was the major route of excretion, accounting for 74-80% and 90-94% in males and females, respectively, following both low- and high-dose and single and repeated dose administration. Time course data revealed that a significant portion of the radioactivity in urine was excreted by 48-72 hours post exposure. Feces were the secondary route of elimination, accounting for 21-24% and 9-10% in males and females, respectively, following both low- and high-dose and single and repeated dose administration. The biliary excretion study determined that approximately 9.5% of the administered dose appeared in bile by 48 hours in low-dose males and females and high-dose females, with approximately 16.5% in high-dose males. Tissue distribution studies indicated that 14C-SL-160(P) is rapidly excreted from the system, with less than 4% (males) and 1% (females) of the administered radioactivity remaining in the tissues seven days after dosing. In general, the pattern of the radiolabel distribution in tissues was similar between the low- and high-dose groups in the single oral dose study and between the low-dose groups in the single and repeated dose groups. Pharmacokinetic studies confirmed the rapid absorption and elimination of 14C-SL-160(P). Following a single oral dose of (14)C-SL-160(P), tmax values (provided only for individual animals; mathematically determined value not provided) ranged from 30 minutes (3/5 animals/sex) or 4 hours (2/5 animals/sex) at 2 mg/kg, while the median tmax was 6 hours in males and 4 hours in females at the 50 mg/kg dose. At 2 and 50 mg/kg, males had slightly longer elimination half times for the blood than females (males: 27 and 36 hours, respectively; females: 18.8 and 33.8 hours, respectively). Likewise, males had a greater AUC (304 and 4440 ug-eq/g hr, respectively) than females (189 and 3080 ug-eq/g hr, respectively).
USEPA/OPPTS; Health Effects Division Records Center Series 361 Science Reviews File R124315 on Flazasulfuron (CAS # 104040-78-0) p.45-6 (November 16, 2005). Available from, as of June 16, 2011: https://www.epa.gov/pesticides/chem_search/hhbp/R124315.pdf
A series of studies were conducted to evaluate the absorption, distribution, metabolism, and excretion of (14)C-SL-160(P)/flazasulfuron/ in groups of 4 or 5 rats (CRL: CD BR VAF/Plus)/sex. In these studies, (14)C-SL-160(P) (CP-1385; 55.8 mCi/mMole; radiolabel purity variously reported as 97.29%, 97.5%. 98.49%., 99.2% and >98%) was administered orally by gavage at doses of 2 or 50 mg/kg or 2 mg/kg . In /one study/, rats had received daily oral gavage doses of nonlabeled SL-160 (Y-920205; 98.9% purity) for 14 days, prior to oral dosing with (14)C-SL-160(P) on Day 15. In the single and repeated dose studies, parent compound was the primary component in the metabolite profiles for urine (males: 19-40%; females: 51-67% of administered dose), with lesser amounts in feces (males and females: 2-5% of administered dose), and bile (males and females: 0.6-2% of administered dose). Including parent compound up to 11 fractions were detected among these matrices. The most prevalent metabolite (expressed as percent of administered dose) were HDTG+TPPG (approximately 6.5-12% in urine, approximately 0.4-2% in feces, and approximately 6.5-14% in bile), with minor amounts < 5%) of DTPU (N-(4,6-Dimethoxy-2-pyrimidinyl)-N-[3-(trifluoromethyl)-2-pyridinyl]urea), HDPU, 6-Methoxy-2-[[3-(trifluoromethyl)-2-pyridinyl]amino]-4-pyrimidinol, TPSA (3-Trifluoromethyl)-2-pyridinesulfonamide), and MTMG. Individually, unidentified fractions generally accounted for less than 2% of the administered dose in the urine and biliary metabolite profile. The fecal metabolite profile had up to 35% unidentified of the recovered dose.
USEPA/OPPTS; Health Effects Division Records Center Series 361 Science Reviews File R124315 on Flazasulfuron (CAS # 104040-78-0) p.45-6 (November 16, 2005). Available from, as of June 16, 2011: https://www.epa.gov/pesticides/chem_search/hhbp/R124315.pdf
