
1. Ertapenem
2. Ertapenem Sodium
3. Invanoz
1. Ertapenem Sodium
2. Ertapenem Monosodium
3. Ertapenem Sodium [usan]
4. Ertapenem Monosodium (90per Cent)
5. 153773-82-1
6. Mk 0826
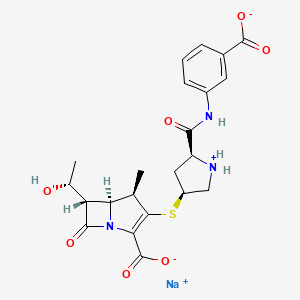
7. Sodium;(4r,5s,6s)-3-[(3s,5s)-5-[(3-carboxylatophenyl)carbamoyl]pyrrolidin-1-ium-3-yl]sulfanyl-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate
8. 2t90ke67l0
9. Mk 826
10. Unii-2t90ke67l0
11. L 749345
12. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 3-(((3s,5s)-5-(((3-carboxyphenyl)amino)carbonyl)-3-pyrrolidinyl)thio)-6-((1r)-1-hydroxyethyl)-4-methyl-7-oxo-, Monosodium Salt, (4r,5s,6s)-
13. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 3-((5-(((3-carboxyphenyl)amino)carbonyl)-3-pyrrolidinyl)thio)-6-((1r)-1-hydroxyethyl)-4-methyl-7-oxo-, Monosodium Salt, (4r-(3(3s*,5s*),4alpha,5beta,6beta(r*)))-
14. Sodium (4r,5s,6s)-3-({(3s,5s)-5-[(3-carboxylatophenyl)carbamoyl]pyrrolidinium-3-yl}sulfanyl)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate
15. Sodium (4r,5s,6s)-3-({(3s,5s)-5-[(m-carboxylatophenyl)carbamoyl]pyrrolidinium-3-yl}sulfanyl)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate
16. Sodium (4r,5s,6s)-3-({(3s,5s)-5-[(m-carboxyphenyl)carbamoyl]-3-pyrrolidinyl}thio)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate
| Molecular Weight | 497.5 g/mol |
|---|---|
| Molecular Formula | C22H24N3NaO7S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 497.12326557 g/mol |
| Monoisotopic Mass | 497.12326557 g/mol |
| Topological Polar Surface Area | 192 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 882 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
* Treatment:
Treatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required:
- intra-abdominal infections;
- community-acquired pneumonia;
- acute gynaecological infections;
- diabetic foot infections of the skin and soft tissue.
* Prevention:
Invanz is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DH03