

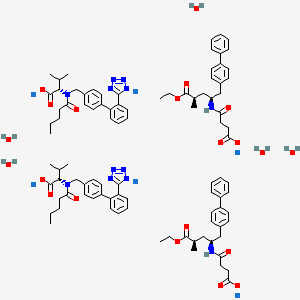

1. 3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate
2. Lcz 696
3. Lcz-696
4. Lcz696
5. Sacubitril And Valsartan Drug Combination
6. Sacubitril And Valsartan Sodium Anhydrous Drug Combination
7. Sacubitril And Valsartan Sodium Hydrate Drug Combination
8. Sacubitril Valsartan Drug Combination
9. Sacubitril Valsartan Sodium Anhydrous
10. Sacubitril Valsartan Sodium Hydrate
11. Sacubitril-valsartan
12. Sacubitril-valsartan Sodium Anhydrous Drug Combination
13. Sacubitril-valsartan Sodium Hydrate Drug Combination
14. Trisodium (3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate) Hemipentahydrate
1. Sacubitril Valsartan Sodium Hydrate
2. Sacubitril Mixture With Valsartan
3. Wb8ft61183
4. Lcz 696
5. Sacubitril Valsartan Sodium Hydrate (jan)
6. Sacubitril Valsartan Sodium Hydrate [jan]
7. Entresto (tn)
8. Unii-wb8ft61183
9. Valsartan Mixture With Ahu-377
10. Ex-a2849
11. Mfcd29477717
12. 3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate
13. Ac-29037
14. Sucabitril Valsartan Sodium Hydrate
15. Trisodium (3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate) Hemipentahydrate
16. D10226
17. Valsartan Ahu-377 Sodium Hemipentahydrate
18. Q27292546
19. L-valine, N-(1-oxopentyl)-n-((2'-(2h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, Compd. With .alpha.-ethyl (.alpha.r,.gamma.s)-.gamma.-((3-carboxy-1-oxopropyl)amino)-.alpha.-methyl(1,1'-biphenyl)-4-pentanoate, Sodium Salt, Hydrate (2:2:6:5)
20. L-valine, N-(1-oxopentyl)-n-((2'-(2h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, Compd. With Alpha-ethyl(alphar,gammas)-gamma-((3-carboxy-1-oxopropyl)amino)-alpha-methyl(1,1'-biphenyl)-4-pentanoate, Sodium Salt, Hydrate (2:2:6:5)
| Molecular Weight | 1916.0 g/mol |
|---|---|
| Molecular Formula | C96H120N12Na6O21 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 29 |
| Rotatable Bond Count | 40 |
| Exact Mass | 1915.8110694 g/mol |
| Monoisotopic Mass | 1914.8077146 g/mol |
| Topological Polar Surface Area | 396 Ų |
| Heavy Atom Count | 135 |
| Formal Charge | 0 |
| Complexity | 1140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 15 |
Entresto is indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction.
Angiotensin Receptor Antagonists
Agents that antagonize ANGIOTENSIN RECEPTORS. Many drugs in this class specifically target the ANGIOTENSIN TYPE 1 RECEPTOR. (See all compounds classified as Angiotensin Receptor Antagonists.)
C09DX04

