

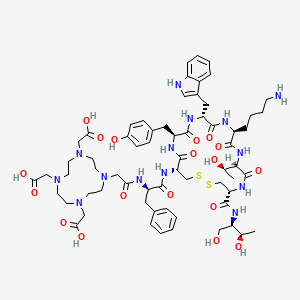

1. (dota(0)-phe(1)-tyr(3))octreotide
2. Dotatoc
3. Smt 487
4. Smt-487
1. Dotatoc
2. Smt-487
3. Smt 487
4. 204318-14-9
5. Chembl408350
6. U194as08hz
7. 173606-11-6
8. Edotreotide [usan]
9. Edotreotide [usan:inn]
10. Unii-u194as08hz
11. Dota-toc Acetate
12. Dota-toc
13. N-acetyl-lys-octreotide
14. Edotreotide [mi]
15. Edotreotide [inn]
16. Schembl1649285
17. Smt487
18. Schembl19712197
19. Dtxsid701021591
20. Ex-a4065
21. Bdbm50165171
22. Hy-106033
23. Cs-0024683
24. Q908790
25. L-cysteinamide, N-((4,7,10-tris(carboxymetnyl)-1,4,7,10-tetraazacyclodec-1-yl)acetyl)-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryptophyl-l-lysyl-l-threonyl-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl)-, Cyclic(2->7)-disulfide
26. L-cysteinamide, N-((4,7,10-tris(carboxymetnyl)-1,4,7,10-tetraazacyclodec-1-yl)acetyl)-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryptophyl-l-lysyl-l-threonyl-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl)-, Cyclic(2-7)-disulfide
27. N-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacycoldodec-1-yl)acetyl-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryprophyl-l-lysyl-l-threonyl-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl)-l Cysteinamide Cyclic (2-7)-disulfide
28. N-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacycoldodec-1-yl)acetyl-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryprophyl-l-lysyl-l-threonyl-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl)-l-cysteinamide Cyclic (2->7)-disulfide
29. N-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacycoldodec-1-yl)acetyl-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryprophyl-l-lysyl-l-threonyl-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl)-l-cysteinamide Cyclic (2-7)-disulfide
| Molecular Weight | 1421.6 g/mol |
|---|---|
| Molecular Formula | C65H92N14O18S2 |
| XLogP3 | -7.2 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 25 |
| Rotatable Bond Count | 26 |
| Exact Mass | 1420.61554449 g/mol |
| Monoisotopic Mass | 1420.61554449 g/mol |
| Topological Polar Surface Area | 531 Ų |
| Heavy Atom Count | 99 |
| Formal Charge | 0 |
| Complexity | 2620 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
This medicinal product is for diagnostic use only.
After radiolabelling with gallium (68Ga) chloride solution, the solution of gallium (68Ga) edotreotide obtained is indicated for Positron Emission Tomography (PET) imaging of somatostatin receptor overexpression in adult patients with confirmed or suspected well-differentiated gastro-enteropancreatic neuroendocrine tumours (GEP-NET) for localizing primary tumours and their metastases.
Radiopharmaceuticals
Compounds that are used in medicine as sources of radiation for radiotherapy and for diagnostic purposes. They have numerous uses in research and industry. (Martindale, The Extra Pharmacopoeia, 30th ed, p1161) (See all compounds classified as Radiopharmaceuticals.)
V09IX
