

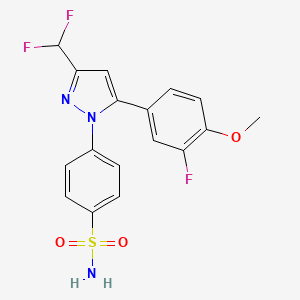

1. 4-(3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1h-pyrazol-1-yl)benzenesulfonamide
2. Deramaxx
3. Nsc 758935
1. 169590-41-4
2. Deramaxx
3. 4-(3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1h-pyrazol-1-yl)benzenesulfonamide
4. Sc 46
5. Sc 046
6. Sc 59046
7. Sc-59046
8. Deracoxib [usan]
9. 4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1h-pyrazol-1-yl]benzenesulfonamide
10. Vx29jb5xwv
11. Nsc-758935
12. 4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)pyrazol-1-yl]benzenesulfonamide
13. Chembl28636
14. Chebi:73032
15. Deracoxib (usan)
16. Ncgc00095312-01
17. Dsstox_cid_25975
18. Dsstox_rid_81266
19. Dsstox_gsid_45975
20. Deram
21. Cas-169590-41-4
22. Deracoxib [usan:inn]
23. Unii-vx29jb5xwv
24. Deracoxibum
25. Deracoxib-[d4]
26. Deracoxib [inn]
27. Deracoxib [mi]
28. Spectrum2_000521
29. Spectrum3_001677
30. Spectrum4_001227
31. Spectrum5_001644
32. Deracoxib [who-dd]
33. Schembl24645
34. Bspbio_003493
35. Kbiogr_001694
36. Spbio_000501
37. Deracoxib [green Book]
38. Dtxsid4045975
39. Kbio3_002713
40. Ex-a887
41. Hms2093m12
42. Hms3886o18
43. Pharmakon1600-01505222
44. Zinc607803
45. Amy31402
46. Bcp04295
47. Tox21_111503
48. Bdbm50057583
49. Ccg-39562
50. Mfcd09837763
51. Nsc758935
52. S5711
53. Sc-046
54. Akos016009604
55. Tox21_111503_1
56. Bcp9000598
57. Db11395
58. Nsc 758935
59. 4-[5-(3-fluoro-4-methoxyphenyl)-3-(difluoromethyl)-1h-pyrazol-1-yl]benzenesulfonamide
60. Ncgc00095312-02
61. Ncgc00095312-04
62. 1-propanol, 2,2-dimethyl-, 1-benzoate
63. Ac-33031
64. As-19558
65. Hy-17509
66. Bcp0726000080
67. Sbi-0206729.p001
68. Ft-0665858
69. D03689
70. Ab01563051_01
71. Sr-05000001985
72. J-010565
73. Q5261287
74. Sr-05000001985-1
75. Brd-k68558722-001-02-4
76. 4-[3-difluoromethyl-5-(3-fluoro-4-methoxy-phenyl)-pyrazol-1-yl]-benzenesulfonamide
77. Benzenesulfonamide, 4-(3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1h-pyrazol-1-yl)-
| Molecular Weight | 397.4 g/mol |
|---|---|
| Molecular Formula | C17H14F3N3O3S |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 397.07079698 g/mol |
| Monoisotopic Mass | 397.07079698 g/mol |
| Topological Polar Surface Area | 95.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 596 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
