


1. 9350, Gs
2. Gs 9350
3. Gs-9350
4. Gs9350
5. Tybost
1. 1004316-88-4
2. Cobicistat (gs-9350)
3. Gs-9350
4. Tybost
5. Gs 9350
6. Cobicistat,gs-9350
7. Unii-lw2e03m5pg
8. Chebi:72291
9. Lw2e03m5pg
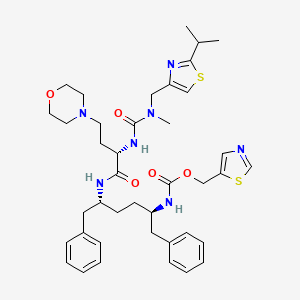
10. 1,3-thiazol-5-ylmethyl [(2r,5r)-5-{[(2s)-2-({[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)carbamoyl}amino)-4-(morpholin-4-yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate
11. 1,3-thiazol-5-ylmethyl N-[(2r,5r)-5-[[(2s)-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]-4-morpholin-4-ylbutanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate
12. Thiazol-5-ylmethyl ((2r,5r)-5-((s)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-morpholinobutanamido)-1,6-diphenylhexan-2-yl)carbamate
13. 1,3-thiazol-5-ylmethyl N-[(2r,5r)-5-[(2s)-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}-4-(morpholin-4-yl)butanamido]-1,6-diphenylhexan-2-yl]carbamate
14. 2,7,10,12-tetraazatridecanoic Acid, 12-methyl-13-(2-(1-methylethyl)-4-thiazolyl)-9-(2-(4-morpholinyl)ethyl)-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, (3r,6r,9s)-
15. Cobicistat [usan]
16. Cobicistat [usan:inn]
17. Cobicistatum
18. 2,7,10,12-tetraazatridecanoic Acid, 12-methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, (3r,6r,9s)-
19. Thiazol-5-ylmethyl (2r,5r)-5-((s)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-morpholinobutanamido)-1,6-diphenylhexan-2-ylcarbamate
20. Tybost (tn)
21. Cobicistat [mi]
22. Cobicistat [inn]
23. Cobicistat [jan]
24. Cobicistat; Gs-9350
25. Cobicistat [vandf]
26. Cobicistat [who-dd]
27. Cobicistat On Silicon Dioxide
28. Cobicistat (jan/usan/inn)
29. Gtpl7535
30. Schembl2736227
31. Chembl2095208
32. Cobicistat [orange Book]
33. Cobicistat, (r,r,s)-
34. Dtxsid00143269
35. Evotaz Component Cobicistat
36. Genvoya Component Cobicistat
37. Symtuza Component Cobicistat
38. Bcp06630
39. Ex-a2578
40. Rezolsta Component Cobicistat
41. Stribild Component Cobicistat
42. Bdbm50447471
43. Mfcd18251449
44. Prezcobix Component Cobicistat
45. S2900
46. Zinc85537014
47. Cobicistat Component Of Evotaz
48. Akos025404908
49. Cobicistat Component Of Genvoya
50. Cobicistat Component Of Symtuza
51. Ccg-270459
52. Cobicistat Component Of Stribild
53. Cs-0742
54. Db09065
55. Cobicistat Component Of Prezcobix
56. Ncgc00386235-04
57. Ncgc00386235-06
58. Ac-28961
59. As-17061
60. Hy-10493
61. Sw219553-1
62. D09881
63. Ab01566899_01
64. Q5138908
65. (1,3-thiazol-5-yl)methyl (5s,8r,11r)-8,11-dibenzyl-2-methyl-5-(2-(morpholin-4-yl)ethyl)-1-(2-(propan-2-yl)-1,3-thiazol-4-yl)-3,6-dioxo-2,4,7,12-tetraazatridecan-13-oate
66. 1,3-thiazol-5-ylmethyl ((2r,5r)-5-(((2s)-2-((methyl((2-(propan-2-yl)-1,3-thiazol-4-yl)methyl)carbamoyl)amino)-4-(morpholin-4-yl)butanoyl)amino)-1,6-diphenylhexan-2-yl)carbamate
67. Thiazol-5-ylmethyl ((1r,4r)-1-benzyl-4-({(2s)-2-((methyl{(2-(1-methylethyl)thiazol-4-yl)methyl}carbamoyl)amino)-4-(morpholin-4-yl)butanoyl}amino)-5-phenylpentyl)carbamate
68. Thiazol-5-ylmethyl N-[(1r,4r)-1-benzyl-4-[[(2s)-2-[[(2-isopropylthiazol-4-yl)methyl-methyl-carbamoyl]amino]-4-morpholino-butanoyl]amino]-5-phenyl-pentyl]carbamate
| Molecular Weight | 776.0 g/mol |
|---|---|
| Molecular Formula | C40H53N7O5S2 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 20 |
| Exact Mass | 775.35496016 g/mol |
| Monoisotopic Mass | 775.35496016 g/mol |
| Topological Polar Surface Area | 195 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications.
FDA Label
Tybost is indicated as a pharmacokinetic enhancer of atazanavir 300 mg once daily or darunavir 800 mg once daily as part of antiretroviral combination therapy in human immunodeficiency virus-1 (HIV-1) infected adults and adolescents aged 12 years and older:
- weighing at least 35 kg coadministered with atazanavir or
- weighing at least 40 kg coadministered with darunavir.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
V03AX03
J05AR14
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AX - Other therapeutic products
V03AX03 - Cobicistat
Absorption
Median peak plasma concentrations were observed at 3.5 hours post-dose.
Route of Elimination
With single dose administration of [14C] cobicistat after multiple dosing of cobicistat for six days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively.
Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation.
The terminal plasma half-life of cobicistat is approximately 3 to 4 hours.
Cobicistat is a mechanism-based inhibitor of cytochrome P450 3A (CYP3A) isoforms. Inhibition of CYP3A-mediated metabolism by cobicistat increases the systemic exposure of CYP3A substrates atazanavir and darunavir and therefore enables increased anti-viral activity at a lower dosage. Cobicistat does not have any anti-HIV activity on its own.
