

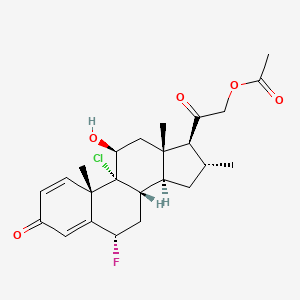

1. Clocortolone
1. 4258-85-9
2. Sh 818
3. Clocortolone Acetate [usan]
4. Clocortolone 21-acetate
5. 85061htr8t
6. Sh-818
7. Clocortolone Acetate (usan)
8. [2-[(6s,8s,9r,10s,11s,13s,14s,16r,17s)-9-chloro-6-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-7,8,11,12,14,15,16,17-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
9. Unii-85061htr8t
10. Schembl618722
11. Chembl2106011
12. Chebi:177410
13. Clocortolone 21-acetate [mi]
14. Db14652
15. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-chloro-6-fluoro-11-hydroxy-16-methyl-, (6.alpha.,11.beta.,16.alpha.)-
16. D03541
17. Q27269574
18. [2-[(6s,8s,9r,10s,11s,13s,14s,16r,17s)-9-chloro-6-luoro-11-hydroxy-10,13,16-trimethyl-3-oxo-7,8,11,12,14,15,16,17-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
19. 9-chloro-6.alpha.-fluoro-11.beta.,21-dihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione 21-acetate
20. 9-chloro-6alpha-fluoro-11beta,21-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 21-acetate
21. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-chloro-6-fluoro-11-hydroxy-16-methyl-, (6 Alpha,11 Beta, 16 Alpha)-
22. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-chloro-6-fluoro-11-hydroxy-16-methyl-, (6alpha,11beta,16alpha)-
| Molecular Weight | 452.9 g/mol |
|---|---|
| Molecular Formula | C24H30ClFO5 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 452.1765799 g/mol |
| Monoisotopic Mass | 452.1765799 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 898 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
