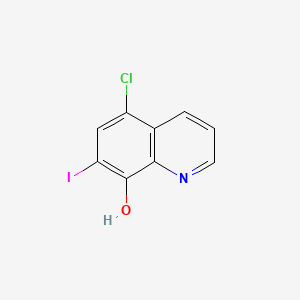

1. 5 Chloro 7 Iodo 8 Quinolinol
2. 5-chloro-7-iodo-8-quinolinol
3. Chinoform
4. Chloroiodoquine
5. Entero Septol
6. Entero Vioform
7. Entero-septol
8. Entero-vioform
9. Enteroquinol
10. Enteroseptol
11. Enterovioform
12. Iodochlorhydroxyquin
13. Iodochloroxyquinoline
14. Vioform
1. 130-26-7
2. 5-chloro-7-iodoquinolin-8-ol
3. Iodochlorhydroxyquin
4. Chinoform
5. Chloroiodoquin
6. 5-chloro-7-iodo-8-quinolinol
7. Chloroiodoquine
8. 5-chloro-8-hydroxy-7-iodoquinoline
9. Vioform
10. Iodochloroxyquinoline
11. Cliquinol
12. Iodochlorohydroxyquinoline
13. Chlorojodochin
14. Enteroquinol
15. Iodochloroquine
16. Iodochloroxine
17. Iodoenterol
18. 7-iodo-5-chloroxine
19. Entero-vioform
20. Iodochlorhydroxyquinoline
21. 5-chloro-7-iodo-8-hydroxyquinoline
22. Iodoxyquinoline
23. Alchloquin
24. Barquinol
25. Budoform
26. Cifoform
27. Dermaform
28. Dioquinol
29. Domeform
30. Eczecidin
31. Enteroseptol
32. Enterozol
33. Enterseptol
34. Entrokin
35. Iodenterol
36. Lekosept
37. Mycoquin
38. Quinambicide
39. Quinoform
40. Rheaform
41. Amebil
42. Amoenol
43. Bactol
44. Emaform
45. Nioform
46. Rometin
47. Entero-bioform
48. Iodochlorohydroxyquin
49. Hi-enterol
50. Iodochlorhydroxyquinol
51. Hydriodide-enterol
52. Quin-o-creme
53. Entero-septol
54. Enterum Locorten
55. 5-chloro-7-iodo-quinolin-8-ol
56. Domeform-hc
57. 8-quinolinol, 5-chloro-7-iodo-
58. 7-iodo-5-chloro-8-hydroxyquinoline
59. Clioquinolum
60. Chloro-8-hydroxyiodoquinoline
61. Quinoform (antiseptic)
62. Vioform N.n.r.
63. Quinoline, Chloro-8-hydroxyiodo-
64. 5-chlor-7-jod-8-hydroxy-chinolin
65. Rheaform Boluses
66. Iodochlorhydroxyquinolone
67. Nsc-3531
68. Chinoformum
69. Cliochinolum
70. 7bhq856ej5
71. Enterovalodon
72. Alioform
73. Enteritan
74. Jodchloroxychinolinum
75. Oralcer
76. Chebi:74460
77. Corque
78. Cortin
79. Iodochloroxychinolinum
80. Cremo-quin
81. Entero-bio Form
82. Entero-vioformio
83. Ala-quin
84. Uad Lotion
85. Nsc-74938
86. Ncgc00016391-05
87. Cliochinolo
88. Cas-130-26-7
89. Cliochinolo [dcit]
90. Tg2-36-2
91. Caswell No. 193
92. Dsstox_cid_2837
93. Formtone-hc
94. Dsstox_rid_76751
95. Pbt-1
96. Dsstox_gsid_22837
97. Quinoform (van)
98. Clioquinolum [inn-latin]
99. Loquinol
100. Vioformio
101. Hi-eneterol
102. Iodochlorhydroxyquin Cream
103. Vioform-hydrocortisone Mild
104. 22112-03-4
105. Smr000058282
106. Rheaform Boluses (veterinary)
107. Ccris 6050
108. Hsdb 6843
109. 5-chlor-7-jod-8-hydroxy-chinolin [german]
110. Sr-01000002987
111. Nsc 3531
112. Einecs 204-984-4
113. Epa Pesticide Chemical Code 024001
114. Brn 0153637
115. Unii-7bhq856ej5
116. Linolasept
117. Clioquinol [usp:inn:ban]
118. Cloquinol
119. Ai3-16451
120. Clioquinol (cq)
121. Component Of Hyquin
122. Mfcd00006787
123. Nsc 74938
124. Nystaform (salt/mix)
125. Clioquinol (usp/inn)
126. Clioquinol [inn]
127. Component Of Formtone-hc
128. Component Of Heb-cort V
129. Prestwick0_000886
130. Prestwick1_000886
131. Prestwick2_000886
132. Prestwick3_000886
133. Clioquinol [hsdb]
134. Formtone-hc (salt/mix)
135. Clioquinol [vandf]
136. Chembl497
137. Ec 204-984-4
138. Cid_2788
139. Clioquinol [mart.]
140. Schembl3967
141. Clioquinol [usp-rs]
142. Clioquinol [who-dd]
143. Nciopen2_009062
144. Oprea1_438281
145. Bspbio_000672
146. Bspbio_002466
147. 5-21-03-00294 (beilstein Handbook Reference)
148. Ksc-8-192
149. Mls000069389
150. Mls002454410
151. Spectrum1505114
152. Spbio_002891
153. Bpbio1_000740
154. Clioquinol [orange Book]
155. Component Of Hyquin (salt/mix)
156. Dtxsid7022837
157. Bdbm32188
158. Iodochlorhydroxyquin [mi]
159. Clioquinol [ep Monograph]
160. Nsc3531
161. Wln: T66 Bnj Gg Ii Jq
162. Clioquinol [usp Monograph]
163. Hms1570b14
164. Hms1648j07
165. Hms2093i12
166. Hms2097b14
167. Hms2230i20
168. Hms3372j20
169. Hms3714b14
170. Kuc105859n
171. Pharmakon1600-01505114
172. Albb-031653
173. Component Of Cort-quin (salt/mix)
174. Zinc6409735
175. Tox21_110416
176. Tox21_200291
177. Nsc759822
178. Nystaform Component Clioquinol
179. S4601
180. Stk399761
181. 5-chloro-8-hydroxy-7-iodo-quinoline
182. Component Of Heb-cort V (salt/mix)
183. Akos000120779
184. Tox21_110416_1
185. Ac-6792
186. Ccg-213339
187. Db04815
188. Fd10468
189. Nsc-759822
190. Clioquinol Component Of Nystaform
191. Smp1_000073
192. Iodochlorhydroxyquin [green Book]
193. Ncgc00016391-01
194. Ncgc00016391-02
195. Ncgc00016391-03
196. Ncgc00016391-04
197. Ncgc00016391-06
198. Ncgc00016391-07
199. Ncgc00016391-08
200. Ncgc00016391-09
201. Ncgc00016391-10
202. Ncgc00016391-13
203. Ncgc00021665-03
204. Ncgc00021665-04
205. Ncgc00021665-05
206. Ncgc00257845-01
207. S4601 5-chloro-8-hydroxy-7-iodoquin
208. As-14597
209. Bc167259
210. Hy-14603
211. Sbi-0206782.p001
212. Db-041956
213. Ab00384254
214. Ft-0603209
215. 4-stearylamino-phenyl-trimethylam. Metilsulf.
216. A16461
217. Clioquinol, Vetranal(tm), Analytical Standard
218. Component Of Vioform-hydrocortisone (salt/mix)
219. D03538
220. Ab00384254_17
221. 5-chloro-7-iodo-8-quinolinol, >=95.0% (hplc)
222. Cu-01000000767-2
223. Q-200875
224. Q5134338
225. Sr-01000002987-2
226. Sr-01000002987-3
227. Sr-01000002987-4
228. Brd-k09255212-001-04-2
229. Z2768770393
230. Clioquinol, British Pharmacopoeia (bp) Reference Standard
231. Clioquinol, European Pharmacopoeia (ep) Reference Standard
232. Clindamycin Phosphate, Antibiotic For Culture Media Use Only
233. Clioquinol, United States Pharmacopeia (usp) Reference Standard
234. Cql
| Molecular Weight | 305.50 g/mol |
|---|---|
| Molecular Formula | C9H5ClINO |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 304.91044 g/mol |
| Monoisotopic Mass | 304.91044 g/mol |
| Topological Polar Surface Area | 33.1 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Amebicides; Anti-Infective Agents, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Clioquinol is used topically in the treatment of tinea pedis, tinea cruris, and other skin infections caused by dermatophytic fungi (ringworm).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
MEDICATION (VET): has been used as an intestinal anti-infective.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 796
Topical anti-infective; anti-amebic
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 796
For more Therapeutic Uses (Complete) data for CLIOQUINOL (7 total), please visit the HSDB record page.
Although clioquinol previously was used in the treatment of diaper rash (diaper dermatitis), use of the drug in children currently is not recommended, and use in those younger than 2 years of age is contraindicated.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
Clioquinol generally appears to be well tolerated following topical application to the skin. Local irritation, rash, and sensitivity reactions have been reported occasionally. If any of these effects occurs, the drug should be discontinued and a physician consulted. Cross sensitivity with other hydroxyquinoline and quinoline derivatives (eg, certain antimalarials) and, occasionally, to iodides can occur. Clioquinol can stain the skin, and discoloration of the hair and nails has been reported rarely. Prolonged use of the drug can result in overgrowth of nonsusceptible organisms, which may require appropriate therapy. When clioquinol is used topically in fixed combination with hydrocortisone, the usual precautions associated with topical corticosteroid therapy should be observed.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
Because of an association between oculotoxic/neurotoxic effects (eg, optic neuritis, optic atrophy, subacute myelo-optic neuropathy) and oral clioquinol therapy (usually at high dosages for prolonged periods) and the availability of effective alternative topical antifungals, use of clioquinol in children is not recommended, and use in those younger than 2 years of age is contraindicated.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
Percutaneously absorbed clioquinol, which contains iodine, may interfere with certain thyroid function tests (eg, protein-bound iodine); therefore, the manufacturer recommends that at least 1 month elapse between discontinuance of topical therapy with the drug and performance of these tests. Clioquinol also may produce false positive results in the ferric chloride test for phenylketonuria when the drug is present in urine or the diaper.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
For more Drug Warnings (Complete) data for CLIOQUINOL (8 total), please visit the HSDB record page.
Used as a topical antifungal treatment.
Clioquinol is a broad-spectrum antibacterial with antifungal properties. Application of clioquinol to extensive or eroded areas of the skin may lead to increased protein-bound iodine (PBI) levels within 1 week. In addition, elevated PBI levels may occur when relatively small areas of the skin are treated with clioquinol for more than 1 week.
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AH - Quinoline derivatives
D08AH30 - Clioquinol
D - Dermatologicals
D09 - Medicated dressings
D09A - Medicated dressings
D09AA - Medicated dressings with antiinfectives
D09AA10 - Clioquinol
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AC - Quinoline derivatives
G01AC02 - Clioquinol
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AA - Hydroxyquinoline derivatives
P01AA02 - Clioquinol
S - Sensory organs
S02 - Otologicals
S02A - Antiinfectives
S02AA - Antiinfectives
S02AA05 - Clioquinol
Absorption
Topical absorption is rapid and extensive, especially when the skin is covered with an occlusive dressing or if the medication is applied to extensive or eroded areas of the skin. Clioquinol is absorbed through the skin in sufficient amounts to affect thyroid function tests.
Clioquinol is absorbed systemically following topical application to the skin. In 2 studies in which clioquinol combined with a corticosteroid was applied topically to the skin as a cream or ointment, it was estimated that about 2-3% of the dose was absorbed systemically. However, in another study in which the drug was applied alone to the skin as a 3% cream and covered with an occlusive wrap for 12 hr, it was estimated that about 40% of the dose was absorbed percutaneously during this period.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2178
Patients with widespread dermatitis were treated with 15-20 g of 3% clioquinol ointment to 40% of the body area twice daily. The serum concentration increased to 0.8-1.2 ug/ml within 4 hr of application. In one patient, 15-20 mg was excreted in the urine daily mainly in the form of conjugated metabolites. This skin treatment resulted in a urinary excretion somewhat less than is obtained after a daily oral dose of 25 mg (one tablet) of clioquinol. Thus, 3-4% of the applied clioquinol was absorbed. 25% was excreted in the urine.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 363
Clioquinol has known human metabolites that include Clioquinol O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
11-14 hr
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 363
Clioquinol is bacteriostatic, however, the precise mechanism of its action is unknown.