

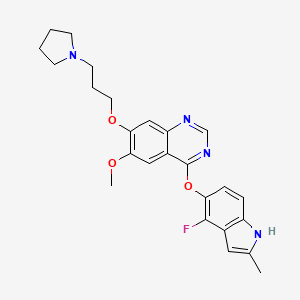

1. 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline
2. 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline (2z)-but-2-enedioate
3. 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline (2z)-but-2-enedioate (1:1)
4. Azd 2171
5. Azd-2171
6. Azd-2171 Maleate
7. Azd2171
8. Azd2171 Maleate
9. Cediranib Maleate
10. Recentin
1. 288383-20-0
2. Recentin
3. Azd2171
4. Cedirannib
5. Cediranib (azd2171)
6. Azd 2171
7. Azd-2171
8. 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline
9. Cediranib Free Base
10. 4-(4-fluoro-2-methylindol-5-yloxy)-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline
11. Cediranib (azd217)
12. Azd-2171 Maleate
13. 4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline
14. Nqu9ipy4k9
15. Zd-2171
16. 4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline
17. Chembl491473
18. 288383-20-0 (free Base)
19. Nsc-732208
20. 4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline
21. Cediranib [inn]
22. Quinazoline, 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-(1-pyrrolidinyl)propoxy)-
23. Kinome_3318
24. Cediranib (usan/inn)
25. Unii-nqu9ipy4k9
26. Cediranib [usan:inn:ban]
27. 4-(4-fluoro-2-methylindol-5-yloxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline
28. 4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline
29. Cediranib,azd2171
30. Cediranib Dihydrochloride
31. Azd2171, Cediranib
32. Cediranib [usan]
33. Cediranib - Azd2171
34. Cediranib [mart.]
35. Cediranib [who-dd]
36. Schembl63147
37. Mls006010063
38. Gtpl5664
39. Chebi:94782
40. Dtxsid10183035
41. Bcpp000295
42. Hms3654g05
43. Hms3674g17
44. Hms3744o21
45. Amy16021
46. Bcp01378
47. Ex-a2039
48. Zinc3948085
49. Bdbm50331096
50. Mfcd09954115
51. Nsc755606
52. Nsc800069
53. S1017
54. Akos005145767
55. Bcp9000500
56. Ccg-264679
57. Cs-0119
58. Db04849
59. Es-0052
60. Nsc-755606
61. Nsc-800069
62. Sb16536
63. Zd 2171
64. Ncgc00263097-01
65. Ncgc00263097-09
66. Ac-25033
67. Hy-10205
68. Quinazoline,4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-[3-(1-pyrrolidinyl)propoxy]-
69. Smr004701223
70. Ft-0751000
71. Sw219261-1
72. Ec-000.2328
73. A24280
74. D08881
75. Q-101399
76. Q5057052
77. Brd-k86930074-001-01-9
78. 4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxy-7-(3-pyrrolidin-1-yl-propoxy)-quinazoline
79. Av3
| Molecular Weight | 450.5 g/mol |
|---|---|
| Molecular Formula | C25H27FN4O3 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 450.20671890 g/mol |
| Monoisotopic Mass | 450.20671890 g/mol |
| Topological Polar Surface Area | 72.5 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 625 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of liver cancer, advanced non-small cell lung cancer (NSCLC), advanced colorectal cancer (CRC) and other solid tumors.
Cediranib is a once-daily, orally available, highly potent and selective VEGF signalling inhibitor that inhibits all three VEGF receptors. The preclinical profile of Cediranib indicates that it has the potential to be the 'best in class' VEGF signalling inhibitor. Phase I data indicate that Cediranib is generally well tolerated, with the most common dose related adverse events being diarrhoea, hoarseness, headache and hypertension.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EK - Vascular endothelial growth factor receptor (vegfr) tyrosine kinase inhibitors
L01EK02 - Cediranib
Absorption
Available following oral administration.
12 to 35 hours
Cediranib inhibits vacular endothelial growth factor (VEGF) receptor tyrosine kinase (RTK). By forming a blockade at the VEGF receptors, Cediranib limits the growth of new blood vessels, which are essential to supporting tumor growth. Thus, lacking sufficient blood supply, tumor cells become starved for nutrients, slowing or halting growth and potentially improving the efficacy of other treatments. Preclinical evidence indicated that the drug had a high affinity at these sites, and was well tolerated and efficacious in animal studies.
