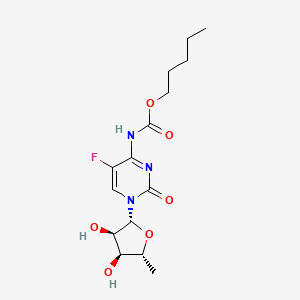

1. N(4)-pentyloxycarbonyl-5'-deoxy-5-fluorocytidine
2. Xeloda
1. 154361-50-9
2. Xeloda
3. Capiibine
4. Capecitibine
5. Captabin
6. Capecytabine
7. 5'-deoxy-5-fluoro-n-[(pentyloxy)carbonyl]cytidine
8. Ro 09-1978
9. Capecitabine Sun
10. Capecitabine Teva
11. Capecitabine Medac
12. Capecitabine Accord
13. Ro 09-1978/000
14. Ecansya
15. Pentyl (1-((2r,3r,4s,5r)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate
16. Cytidine, 5'-deoxy-5-fluoro-n-[(pentyloxy)carbonyl]-
17. Pentyl 1-(5-deoxy-beta-d-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate
18. Pentyl N-[1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate
19. 5'-deoxy-5-fluoro-n-((pentyloxy)carbonyl)cytidine
20. Ro-091978000
21. Chebi:31348
22. 6804dj8z9u
23. Nsc-759853
24. (1-(5-deoxy-beta-d-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-carbamic Acid Pentyl Ester
25. Pentyl N-{1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl}carbamate
26. Capecitabin
27. Caxeta
28. Xabine
29. Capecitabine [usan]
30. Ro-09-1978000
31. Dsstox_cid_26451
32. Dsstox_rid_81625
33. Pentyl [1-(5-deoxy-beta-d-ribofuranosyl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]carbamate
34. Dsstox_gsid_46451
35. Pentyl 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-ylcarbamate
36. Cytidine, 5'-deoxy-5-fluoro-n-((pentyloxy)carbonyl)-
37. Capecitabina
38. Capecitabinum
39. C15h22fn3o6
40. Capecitabine (xeloda)
41. Smr002530052
42. Xeloda (tn)
43. Cas-154361-50-9
44. N(4)-pentyloxycarbonyl-5'-deoxy-5-fluorocytidine
45. Cpecitabine
46. R340
47. Unii-6804dj8z9u
48. Hsdb 7656
49. Ncgc00164569-01
50. Capecitabine [usan:usp:inn:ban]
51. Rg-340
52. Capecitabine- Bio-x
53. Carbamic Acid, (1-(5-deoxy-beta-d-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-, Pentyl Ester
54. Ro-09-1978
55. Capecitabine [mi]
56. R-340
57. Capecitabine [inn]
58. Capecitabine [jan]
59. Capecitabine [hsdb]
60. Schembl8153
61. Capecitabine [vandf]
62. Chembl1773
63. Capecitabine [mart.]
64. Mls003915642
65. Mls004774137
66. Capecitabine [usp-rs]
67. Capecitabine [who-dd]
68. Capecitabine (jan/usp/inn)
69. Gtpl6799
70. Capecitabine [ema Epar]
71. Dtxsid3046451
72. Capecitabine, Analytical Standard
73. Capecitabine, >=98% (hplc)
74. Ex-a835
75. Bcpp000300
76. Capecitabine [orange Book]
77. Capecitabine [ep Monograph]
78. Capecitabine [usp Impurity]
79. Hy-b0016
80. Xeliri Component Capecitibine
81. Zinc3806413
82. Capecitabine [usp Monograph]
83. Tox21_112198
84. Mfcd00930626
85. S1156
86. Akos015920130
87. Tox21_112198_1
88. Am84502
89. Bcp9000483
90. Bs-1000
91. Ccg-264841
92. Cs-0768
93. Db01101
94. Nsc 759853
95. Ncgc00164569-02
96. Ncgc00164569-05
97. Bc164277
98. 3-(2-hydroxyethyl)thiazoliumbromide
99. Ro-9-1978
100. Ro-09-1978-000
101. D01223
102. N4-pentyloxycarbonyl-5'-deoxy-5-fluorocytidine
103. Ab01274776-01
104. Ab01274776-02
105. Ab01274776_04
106. 361c509
107. 5'-deoxy-5-fluoro-n4-(pentyloxycarbonyl)cytidine
108. 5-deoxy-5-fluoro-n-[(pentyloxy)carbonyl]cytidine
109. Q420207
110. Sr-01000931255
111. 5-deoxy-5-fluoro-n4-[(pentyloxy)carbonyl]cytidine
112. J-700154
113. Q-200788
114. Sr-01000931255-3
115. Brd-k61192372-001-08-9
116. Z1741971721
117. Capecitabine, European Pharmacopoeia (ep) Reference Standard
118. Capecitabine, United States Pharmacopeia (usp) Reference Standard
119. Capecitabine, Pharmaceutical Secondary Standard; Certified Reference Material
120. 958887-39-3
121. Carbamic Acid, (1-(5-deoxy-.beta.-d-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-, Pentyl Ester
122. Pentyl 1-(5-deoxy-.beta.-d-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate
| Molecular Weight | 359.35 g/mol |
|---|---|
| Molecular Formula | C15H22FN3O6 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 359.14926359 g/mol |
| Monoisotopic Mass | 359.14926359 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Capecitabine |
| PubMed Health | Capecitabine (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.The chemical name for capecitabine is 5& |
| Active Ingredient | Capecitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 500mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Teva Pharms Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Xeloda |
| PubMed Health | Capecitabine (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.The chemical name for capecitabine is 5& |
| Active Ingredient | Capecitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 500mg |
| Market Status | Prescription |
| Company | Hoffmann La Roche |
| 3 of 4 | |
|---|---|
| Drug Name | Capecitabine |
| PubMed Health | Capecitabine (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.The chemical name for capecitabine is 5& |
| Active Ingredient | Capecitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 500mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Teva Pharms Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Xeloda |
| PubMed Health | Capecitabine (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.The chemical name for capecitabine is 5& |
| Active Ingredient | Capecitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 500mg |
| Market Status | Prescription |
| Company | Hoffmann La Roche |
Antimetabolites, Antineoplastic Agent
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Capecitabine is indicated as a single agent for adjuvant treatment in patients with Dukes' C colon cancer who have undergone complete resection of the primary tumor when treatment with fluoropyrimidine therapy alone is preferred. Capecitabine was non-inferior to 5-fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Although neither Capecitabine nor combination chemotherapy prolongs overall survival (OS), combination chemotherapy has been demonstrated to improve disease-free survival compared to 5-FU/LV. Physicians should consider these results when prescribing single-agent capecitabine in the adjuvant treatment of Dukes' C colon cancer. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Xeloda (Capecitabine) (June 2006). Available from, as of Nov. 17, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=630
Capecitabine is indicated as first-line treatment of patients with metastatic colorectal carcinoma when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV has not been demonstrated with Capecitabine monotherapy. Use of capecitabine instead of 5-FU/LV in combinations has not been adequately studied to assure safety or preservation of the survival advantage. /Included in US product label/.
US Natl Inst Health; DailyMed. Current Medication Information for Xeloda (Capecitabine) (June 2006). Available from, as of Nov. 17, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=630
Capecitabine in combination with docetaxel is indicated for the treatment of patients with metastatic breast cancer after failure of prior anthracycline-containing chemotherapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Xeloda (Capecitabine) (June 2006). Available from, as of Nov. 17, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=630
Capecitabine monotherapy is also indicated for the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or resistant to paclitaxel and for whom further anthracycline therapy is not indicated, eg, patients who have received cumulative doses of 400 mg/sq m of doxorubicin or doxorubicin equivalents. Resistance is defined as progressive disease while on treatment, with or without an initial response, or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant regimen. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Xeloda (Capecitabine) (June 2006). Available from, as of Nov. 17, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=630
Diarrhea, a dose-limiting and common adverse effect of capecitabine, occurs in 55-67% of patients receiving the drug for metastatic breast cancer or metastatic colorectal cancer, and is severe or life-threatening in 15% of patients. Nausea and vomiting occur in 43-53% and 27-37%, respectively, of patients receiving capecitabine for metastatic breast cancer or metastatic colorectal cancer. Among patients with metastatic breast cancer who developed severe nausea and/or vomiting associated with capecitabine monotherapy, onset of these adverse GI effects was early, usually occurring during the first month of treatment.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 959
Among patients receiving capecitabine alone as adjuvant therapy for stage III colon cancer, diarrhea occurred in 47% of patients and was severe or life-threatening (grade 3 or 4) in 12%; nausea occurred in 34%, and vomiting in 15%, of patients.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 959
Severe adverse GI effects associated with capecitabine may occur more frequently in geriatric patients. Among 21 patients aged 80 years or older receiving capecitabine monotherapy for metastatic breast cancer or metastatic colorectal cancer in clinical trials, severe or life-threatening (grade 3 or 4) diarrhea, nausea, or vomiting occurred in 29, 14, or 10%, respectively. Among 10 patients aged 70-80 years receiving capecitabine in combination with docetaxel for metastatic breast cancer, grade 3 or 4 diarrhea and stomatitis each occurred in 30%.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 959
Capecitabine-induced diarrhea may respond to standard antidiarrheal therapy (eg, loperamide). Patients with severe diarrhea should be closely monitored and given fluid and electrolyte replacement for dehydration as indicated.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 959
For more Drug Warnings (Complete) data for CAPECITABINE (38 total), please visit the HSDB record page.
For the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen. May also be used in combination with docetaxel for the treatment of metastatic breast cancer in patients who have failed to respond to, or recurred or relasped during or following anthracycline-containing chemotherapy. Capecitabine is used alone as an adjuvant therapy following the complete resection of primary tumor in patients with stage III colon cancer when monotherapy with fluroprymidine is preferred. The use or capecitabine in combination regimens for advanced gastric cancer is currently being investigated.
FDA Label
Capecitabine Medac is indicated for the adjuvant treatment of patients following surgery of stage-III (Dukes stage-C) colon cancer .
Capecitabine Medac is indicated for the treatment of metastatic colorectal cancer .
Capecitabine Medac is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
Capecitabine Medac in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline.
Capecitabine Medac is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline-containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
Capecitabine Accord is indicated for the adjuvant treatment of patients following surgery of stage-III (Dukes stage-C) colon cancer .
Capecitabine Accord is indicated for the treatment of metastatic colorectal cancer .
Capecitabine Accord is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
Capecitabine Accord in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline.
Capecitabine Accord is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
Ecansya is indicated for the adjuvant treatment of patients following surgery of stage-III (Dukes stage-C) colon cancer .
Ecansya is indicated for the treatment of metastatic colorectal cancer .
Ecansya is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
Ecansya in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline. Ecansya is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
Capecitabine Teva is indicated for the adjuvant treatment of patients following surgery of stage III (Dukes stage C) colon cancer .
Capecitabine Teva is indicated for the treatment of metastatic colorectal cancer .
Capecitabine Teva is indicated for firstline treatment of advanced gastric cancer in combination with a platinumbased regimen.
Capecitabine Teva in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline. Capecitabine Teva is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
- Xeloda is indicated for the adjuvant treatment of patients following surgery of stage III (Dukes' stage C) colon cancer .
- Xeloda is indicated for the treatment of metastatic colorectal cancer .
- Xeloda is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
- Xeloda in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline. Xeloda is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline-containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
Capecitabine is indicated for the adjuvant treatment of patients following surgery of stage-III (Dukes stage-C) colon cancer .
Capecitabine is indicated for the treatment of metastatic colorectal cancer .
Capecitabine is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
Capecitabine in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline. Capecitabine is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline-containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
Capecitabine is a fluoropyrimidine carbamate with antineoplastic activity indicated for the treatment of metastatic breast cancer and colon cancer. It is an orally administered systemic prodrug that has little pharmacologic activity until it is converted to fluorouracil by enzymes that are expressed in higher concentrations in many tumors. Fluorouracil it then metabolized both normal and tumor cells to 5-fluoro-2-deoxyuridine 5-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP).
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
L01BC06
L01BC06
L01BC06
L01BC06
L01BC06
L01BC06
L01BC06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BC - Pyrimidine analogues
L01BC06 - Capecitabine
Absorption
Readily absorbed through the GI tract (~70%)
Route of Elimination
Capecitabine and its metabolites are predominantly excreted in urine; 95.5% of administered capecitabine dose is recovered in urine. Fecal excretion is minimal (2.6%). The major metabolite excreted in urine is FBAL which represents 57% of the administered dose.About 3% of the administered dose is excreted in urine as unchanged drug.
Capecitabine is readily absorbed from the GI tract; on average, at least 70% of an oral dose of the drug is absorbed. Although in vitro studies have shown that capecitabine is unstable under highly acidic conditions, the drug appears to be absorbed intact immediately upon dissolution without degradation secondary to the acidic pH of the stomach.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
According to the manufacturer, peak plasma concentrations of capecitabine occur in about 1.5 hours, and peak plasma concentrations of fluorouracil, its active drug, occur slightly later at 2 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
In adults with cancer who received a capecitabine dosage of 2510 mg/sq m daily in 2 divided doses, administered approximately 12 hours apart within 30 minutes following the end of a meal, blood samples drawn on day 1 of the treatment cycle showed that peak plasma concentrations of 3.93 and 0.66 ug/mL for capecitabine and fluorouracil, respectively, were achieved in about 2 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
Considerable interindividual variations (ie, exceeding 85%) in peak plasma concentrations and areas under the concentration-time curves (AUCs) have been reported following oral administration of capecitabine.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
For more Absorption, Distribution and Excretion (Complete) data for CAPECITABINE (12 total), please visit the HSDB record page.
Metabolized by thymidine phosphorylase to fluoruracil.
Capecitabine, an anticancer prodrug, is thought to be biotransformed into active 5-fluorouracil (5-FU) by three enzymes. After oral administration, capecitabine is first metabolized to 5'-deoxy-5-fluorocytidine (5'-DFCR) by carboxylesterase (CES), then 5'-DFCR is converted to 5'-deoxy-5-fluorouridine (5'-DFUR) by cytidine deaminase. 5'-DFUR is activated to 5-FU by thymidine phosphorylase. Although high activities of drug metabolizing enzymes are expressed in human liver, the involvement of the liver in capecitabine metabolism is not fully understood. In this study, the metabolism of capecitabine in human liver was investigated in vitro. 5'-DFCR, 5'-DFUR, and 5-FU formation from capecitabine were investigated in human liver S9, microsomes, and cytosol in the presence of the inhibitor of dihydropyrimidine dehydrogenase, 5-chloro-2,4-dihydroxypyridine. 5'-DFCR, 5'-DFUR, and 5-FU were formed from capecitabine in cytosol and in the combination of microsomes and cytosol. Only 5'-DFCR formation was detected in microsomes. The apparent K(m) and V(max) values of 5-FU formation catalyzed by cytosol alone and in combination with microsomes were 8.1 mM and 106.5 pmol/min/mg protein, and 4.0 mM and 64.0 pmol/min/mg protein, respectively. The interindividual variability in 5'-DFCR formation in microsomes and cytosol among 14 human liver samples was 8.3- and 12.3-fold, respectively. Capecitabine seems to be metabolized to 5-FU in human liver. 5'-DFCR formation was exhibited in cytosol with large interindividual variability, although CES is located in microsomes in human liver. In the present study, it has been clarified that the cytosolic enzyme would be important in 5'-DFCR formation, as is CES.
PMID:15205393 Tabata T et al; Drug Metab Dispos 32 (7): 762-7 (2004)
Capecitabine (Xeloda; CAP) is a recently developed oral antineoplastic prodrug of 5-fluorouracil (5-FU) with enhanced tumor selectivity. Previous studies have shown that CAP activation follows a pathway with three enzymatic steps and two intermediary metabolites, 5'-deoxy-5-fluorocytidine (5'-DFCR) and 5'-deoxy-5-fluorouridine (5'-DFUR), to form 5-FU preferentially in tumor tissues. In the present work, all fluorinated compounds present in liver, bile, and perfusate medium of isolated perfused rat liver (IPRL) and in liver, plasma, kidneys, bile, and urine of healthy rats /were investigated/. Moreover, data obtained from rat urine were compared with those from mice and human urine. According to a low cytidine deaminase activity in rats, 5'-DFCR was by far the main product in perfusate medium from IPRL and plasma and urine from rats. Liver and circulating 5'-DFCR in perfusate and plasma equilibrated at the same concentration value in the range 25 to 400 microM, which supports the involvement of es-type nucleoside transporter in the liver. 5'-DFUR and alpha-fluoro-beta-ureidopropionic acid (FUPA) + alpha-fluoro-beta-alanine (FBAL) were the main products in urine of mice, making up 23 to 30% of the administered dose versus 3 to 4% in rat. In human urine, FUPA + FBAL represented 50% of the administered dose, 5'-DFCR 10%, and 5'-DFUR 7%. Since fluorine-19 nuclear magnetic resonance spectroscopy gives an overview of all the fluorinated compounds present in a sample, we observed the following unreported metabolites of CAP: 1) 5-fluorocytosine and its hydroxylated metabolite, 5-fluoro-6-hydroxycytosine, 2) fluoride ion, 3) 2-fluoro-3-hydroxypropionic acid and fluoroacetate, and 4) a glucuroconjugate of 5'-DFCR.
PMID:12386128 Desmoulin F et al; Drug Metab Dispos 30 (11): 1221-9 (2002)
Fluorouracil is catabolized to dihydrofluorouracil (FUH2), a much less toxic metabolite, by dihydropyrimidine dehydrogenase. Dihydropyrimidinase cleaves the pyrimidine ring of dihydrofluorouracil, yielding 5-fluoro-ureido-propionic acid (FUPA), which is then cleaved by beta-ureido-propionase to form alpha-fluoro-beta-alanine (FBAL).
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
45-60 minutes for capecitabine and its metabolites.
The plasma elimination half-life of capecitabine and its metabolites, including the active drug, fluorouracil, is about 45-60 minutes, except for alpha-fluoro-beta-alanine (FBAL), a catabolite of fluorouracil, which has an initial half-life of about 3 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
Capecitabine is a prodrug that is selectively tumour-activated to its cytotoxic moiety, fluorouracil, by thymidine phosphorylase, an enzyme found in higher concentrations in many tumors compared to normal tissues or plasma. Fluorouracil is further metabolized to two active metabolites, 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP), within normal and tumour cells. These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N5-10-methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2'-deaxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, therefore a deficiency of this compound can inhibit cell division. Secondly, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis through the production of fraudulent RNA.
Capecitabine is a prodrug and has little pharmacologic activity until it is converted to fluorouracil, an antimetabolite. Because capecitabine is converted to fluorouracil by enzymes that are expressed at higher concentrations in many tumors than in adjacent normal tissues or plasma, it is thought that high tumor concentrations of the active drug may be achieved with less systemic toxicity. Fluorouracil is metabolized in both normal and tumor cells to 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). Although the precise mechanisms of action of fluorouracil have not been fully elucidated, the main mechanism is thought to be the binding of the deoxyribonucleotide of the drug (FdUMP) and the folate cofactor (N5-10-methylenetetrahydrofolate) to thymidylate synthase (TS) to form a covalently bound ternary complex, which inhibits the formation of thymidylate from 2'-deoxyuridylate, thereby interfering with DNA synthesis. In addition, FUTP can be incorporated into RNA in place of uridine triphosphate (UTP), producing a fraudulent RNA and interfering with RNA processing and protein synthesis. Capecitabine has been shown to be active in xenograft tumors that are resistant to fluorouracil indicating incomplete cross-resistance between the drugs.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 963
In this report, /the authors/ investigated whether apoptosis induced by capecitabine was mediated by the Fas/FasL system. To achieve this goal, a specific in vitro coculture model mixing hepatoma and human colorectal cell line was used. A bystander effect was observed between HepG2 and LS174T cells treated with capecitabine. Besides this, Xeloda showed a 7-fold higher cytotoxicity and markedly stronger apoptotic potential in thymidine phosphorylase (TP)-transfected LS174T-c2 cells. The striking enhancement of thymidylate synthase inhibition that we observed in cells with high TP activity was most probably at the origin of the potentiation of capecitabine antiproliferative efficacy. In addition, this increase of sensitivity was accompanied by a strong overexpression of the CD95-Fas receptor on the cell surface. Both Fas and FasL mRNA expression were triggered after exposing TP+ cells to the drug. This implication of Fas in Xeloda-induced apoptosis was next confirmed by using antagonistic anti-Fas and anti-FasL antibodies that proved to reverse capecitabine antiproliferative activity, thus highlighting the key role that Fas could play in the optimization of an antitumor response to fluoropyrimidine drugs. /The/ data, therefore, show that TP plays a key role in the capecitabine activity and that the Fas/FasL system could be considered as a new determinant for Xeloda efficacy.
PMID:12481413 Ciccolini J et al; Mol Cancer Ther 1 (11): 923-7 (2002)