


1. Cbdv Compound
1. Cannabidivarol
2. 24274-48-4
3. Cbdv
4. Cbd-v
5. Gwp42006
6. I198vbv98i
7. Gwp-42006
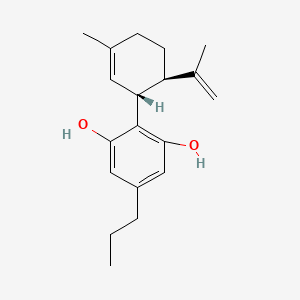
8. 2-[(1r,6r)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-propylbenzene-1,3-diol
9. Cannabidivarin (cbdv)
10. (1'r,2'r)-5'-methyl-2'-(prop-1-en-2-yl)-4-propyl-1',2',3',4'-tetrahydro-[1,1'-biphenyl]-2,6-diol
11. Cannabidivarine
12. (1r-trans)-2-(3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl)-5-propyl-1,3-benzenediol
13. Unii-i198vbv98i
14. Schembl2759238
15. Chembl2387742
16. Chebi:182159
17. Dtxsid801019159
18. Cannabidivarin [nflis-drug]
19. Zinc5844413
20. Bdbm50532215
21. Akos030242161
22. Db14050
23. 1,3-benzenediol, 2-(3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl)-5-propyl-, (1r-trans)-
24. Ac-34111
25. Bc175204
26. C20217
27. Cannabidivarin (cbdv) 100 Microg/ml In Methanol
28. Cannabidivarin (cbdv) 1000 Microg/ml In Methanol
29. Resorcinol, 2-p-mentha-1,8-dien-3-yl-5-propyl-
30. 1,3-benzenediol, 2-((1r,6r)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl)-5-propyl-
31. 2-((1r,6r)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-en-1-yl)-5-propylbenzene-1,3-diol
| Molecular Weight | 286.4 g/mol |
|---|---|
| Molecular Formula | C19H26O2 |
| XLogP3 | 5.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 286.193280068 g/mol |
| Monoisotopic Mass | 286.193280068 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 386 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cannabidivarin does not currently have any FDA or Health Canada approved indications, however in October 2017 CBDV was given orphan designation by the European Medicines Agency for use in Rett Syndrome and again in February 2018 for treatment of Fragile X Syndrome.
Absorption
Like 9-THC, CBDV has low water solubility and poor oral bioavailability (~6% in humans), making oral administration an unfavourable method of delivery. Despite this, CBDV has relatively rapid absorption with peak concentrations seen around 2 h after oral administration in animal pharmacokinetic studies. Orally administered CBDV in mice was found to have a plasma Cmax of 0.47ug/mL and Tmax of 30 minutes, and a brain Cmax of 0.94ug/mL and Tmax of 60 minutes.
Volume of Distribution
Due to its lipophilicity, CBDV has been shown to cross the blood brain barrier.
Significant first-pass metabolism by the liver results in erratic absorption from the GI tract, low bioavailability, and unreliable pharmacokinetics.
Orally administered CBDV in mice was found to have a plasma elimination half life of 222 minutes, and a brain elimination half life of 204 minutes.
The anti-epileptic activity of CBD and CBDV is thought to be modulated by their effects on transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor, which is part of a large family of ion channels that are involved in the onset and progression of several types of epilepsy. CBD and CBDV have been shown to dose-dependently activate and then desensitize TRPV1 as well as TRPV2 and TRPA1 channels. Desensitization of these ion channels is a potential mechanism by which these molecules cause a reduction of neuronal hyperexcitability that contributes to epileptic activity and seizures. CBDV has also been shown to inhibit the activity of diacylglycerol (DAG) lipase-, the primary synthetic enzyme of the endocannabinoid, 2-arachidonoylglycerol (2-AG). The clinical implications of this are unclear however, as this interaction has not been shown to affect CBDV's anticonvulsant activity.
