

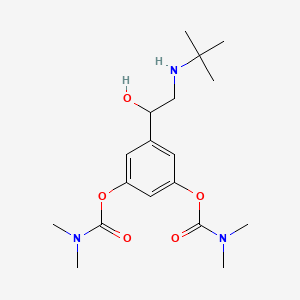

1. 5-(2-(tert-butylamino)-1-hydroxyethyl)-3-phenylene Bis(dimethylcarbamate)
2. Bambuterol Hydrochloride
1. 81732-65-2
2. Bambec
3. Bambuterolum
4. Bambuterol (inn)
5. Chebi:553827
6. [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-(dimethylcarbamoyloxy)phenyl] N,n-dimethylcarbamate
7. Y1850g1ovc
8. (+/-)-bambuterol;kwd-2183
9. (+-)-5-(2-(tert-butylamino)-1-hydroxyethyl)-m-phenylene Bis(dimethylcarbamate)
10. Bambuterolum [latin]
11. Oxeol
12. Bambuterol [inn]
13. Kwd-2183
14. 5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diyl Bis(dimethylcarbamate)
15. (+/-)-5-(2-(tert-butylamino)-1-hydroxyethyl)-m-phenylene Bis(dimethylcarbamate)
16. Terbutaline Bisdimethylcarbamate
17. Bambuterol [inn:ban]
18. Unii-y1850g1ovc
19. Terbutaline Bis(dimethylcarbamate)
20. Bambuterol [mi]
21. Prestwick0_000361
22. Prestwick1_000361
23. Prestwick2_000361
24. Prestwick3_000361
25. Schembl4431
26. Bambuterol [who-dd]
27. Bspbio_000481
28. Mls002153785
29. Spbio_002402
30. Bpbio1_000531
31. Chembl521589
32. Gtpl6601
33. Dtxsid5048550
34. Glxc-25236
35. Hms2089j18
36. Hms2230o15
37. Hms3373n13
38. Bcp21793
39. Bdbm50235800
40. Stk643808
41. Akos005574764
42. Cs-3157
43. Db01408
44. ( Inverted Exclamation Marka)-bambutero
45. ( Inverted Exclamation Marka)-bambuterol
46. Ncgc00179546-01
47. Ncgc00179546-02
48. Ac-35438
49. Hy-17501
50. Smr001233168
51. Sbi-0207028.p001
52. Ft-0602901
53. D07377
54. 732b652
55. A840189
56. L004435
57. Q3633651
58. Sr-05000001470-1
59. Brd-a17462676-003-03-3
60. Brd-a17462676-003-06-6
61. (rs)-5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diyl Bis(dimethylcarbamate)
62. 5-(2-(tert-butylamino)-1-hydroxyethyl)-1,3-phenylene Bis(dimethylcarbamate)
63. [3-[2-(tert-butylamino)-1-oxidanyl-ethyl]-5-(dimethylcarbamoyloxy)phenyl] N,n-dimethylcarbamate
64. N,n-dimethylcarbamic Acid [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-[dimethylamino(oxo)methoxy]phenyl] Ester
| Molecular Weight | 367.4 g/mol |
|---|---|
| Molecular Formula | C18H29N3O5 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 367.21072103 g/mol |
| Monoisotopic Mass | 367.21072103 g/mol |
| Topological Polar Surface Area | 91.3 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 446 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema.
Bambuterol is a long acting beta2-adrenoceptor agonist used in the treatment of asthma. It is a prodrug of terbutaline. Bambuterol causes smooth muscle relaxation, resulting in dilation of bronchial passages.
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03C - Adrenergics for systemic use
R03CC - Selective beta-2-adrenoreceptor agonists
R03CC12 - Bambuterol
Absorption
Bioavailability is 20% following oral administration.
Hepatic, extensive. Further metabolized to terbutaline by plasma cholinesterase.
13 hours for bambuterol and 21 hours for the primary active metabolite terbutaline.
The pharmacologic effects of bambuterol are at least in part attributable to stimulation through beta-adrenergic receptors (beta 2 receptors) of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic AMP. Increased cyclic AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
