

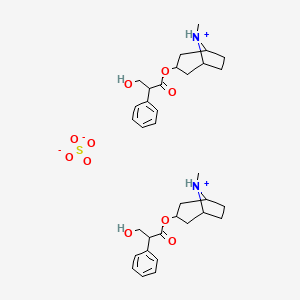

1. Schembl34274
2. Ft-0603494
3. Ft-0656045
| Molecular Weight | 676.8 g/mol |
|---|---|
| Molecular Formula | C34H48N2O10S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 10 |
| Exact Mass | 676.30296691 g/mol |
| Monoisotopic Mass | 676.30296691 g/mol |
| Topological Polar Surface Area | 191 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 415 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 6 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Atropine sulfate |
| Drug Label | Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate in water for injection with sodium chloride sufficient to render the solution isotonic. It is administered parenterally by subcutaneous, intr... |
| Active Ingredient | Atropine sulfate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Atropine sulfate |
| Drug Label | Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate in water for injection with sodium chloride sufficient to render the solution isotonic. It is administered parenterally by subcutaneous, intr... |
| Active Ingredient | Atropine sulfate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Akorn |

