

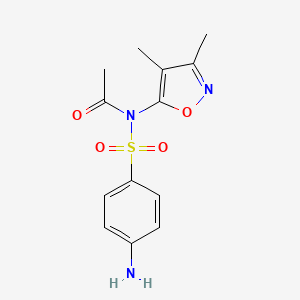

1. Acetyl Sulfisoxazole
1. 80-74-0
2. Acetyl Sulfisoxazole
3. Lipo Gantrisin
4. Acetylsulfafurazole
5. Acetylsulfisoxazole
6. Sulfisoxazole Acetal
7. N-((4-aminophenyl)sulfonyl)-n-(3,4-dimethylisoxazol-5-yl)acetamide
8. Acetylgantrisin
9. N-(4-aminophenyl)sulfonyl-n-(3,4-dimethyl-1,2-oxazol-5-yl)acetamide
10. Acetyl Sulfafurazole
11. Sulfafurazole Acetyl
12. Sulfisoxizole Acetyl
13. N-(3,4-dimethyl-5-isoxazolyl)-n-sulfanilylacetamide
14. Wbt5qh3ked
15. Sulfisoxazole Acetyl [usp]
16. Nsc-759138
17. Acetamide, N-((4-aminophenyl)sulfonyl)-n-(3,4-dimethyl-5-isoxazolyl)-
18. Acetylsulfisoxazole (jan)
19. Sulfisoxazole Acetyl (usp)
20. Acetylsulfisoxazole [jan]
21. N-3,4-dimethylisoxazol-5-yl-n-sulfanilylacetamide
22. Acetamide, N-[(4-aminophenyl)sulfonyl]-n-(3,4-dimethyl-5-isoxazolyl)-
23. Sulfisoxazoleacetyl(200mg)
24. Unii-wbt5qh3ked
25. Einecs 201-305-3
26. N-3,4-dimethylisoxazol-5-yl-n-sulphanilylacetamide
27. N-[(4-aminophenyl)sulfonyl]-n-(3,4-dimethylisoxazol-5-yl)acetamide
28. Sulfisoxazole-acetyl
29. Lipo Gantrisin (tn)
30. Dsstox_cid_3620
31. Dsstox_rid_97551
32. Dsstox_gsid_23620
33. Schembl41727
34. Zinc2114
35. Chembl1200910
36. Dtxsid4023620
37. Acetyl Sulfisoxazole [mi]
38. Chebi:135975
39. Amy37432
40. N-(4-aminophenyl)sulfonyl-n-(3,4-dimethylisoxazol-5-yl)acetamide
41. Sulfisoxazole Acetyl [vandf]
42. Tox21_113660
43. Acetyl Sulfafurazole [mart.]
44. Mfcd00072139
45. Sulfafurazole Acetyl [who-dd]
46. Sulfisoxazole Acetyl [usp-rs]
47. Db14033
48. Nsc 759138
49. Cas-80-74-0
50. Ncgc00249884-01
51. Sulfisoxazole Acetyl [orange Book]
52. Hy-107923
53. Sulfisoxazole Acetyl [usp Monograph]
54. B4954
55. Cs-0030871
56. Eryzole Component Sulfisoxazole Acetyl
57. D05956
58. Pediazole Component Sulfisoxazole Acetyl
59. Sulfisoxazole Acetyl Component Of Eryzole
60. A915833
61. Ilosone Sulfa Component Sulfisoxazole Acetyl
62. Sulfisoxazole Acetyl Component Of Pediazole
63. Q27292550
64. Sulfisoxazole Acetyl Component Of Ilosone Sulfa
65. Acetamide, N-(3,4-dimethyl-5-isoxazolyl)-n-sulfanilyl-
66. N-[(4-aminophenyl)sulfonyl]-n-(3,4-dimethyl-5-isoxazolyl)acetamide
67. N-acetyl-4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamide #
68. N-[(4-aminophenyl)sulfonyl]-n-(3,4-dimethyl-5- Isoxazolyl)acetamide
| Molecular Weight | 309.34 g/mol |
|---|---|
| Molecular Formula | C13H15N3O4S |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 309.07832714 g/mol |
| Monoisotopic Mass | 309.07832714 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 482 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Acute, recurrent or chronic urinary tract infections (primarily pyelonephritis, pyelitis and cystitis) due to susceptible organisms (usually Escherichia coli, Klebsiella-Enterobacter, staphylococcus, Proteus mirabilis and, less frequently, Proteus vulgaris) in the absence of obstructive uropathy or foreign bodies Meningococcal meningitis where the organism has been demonstrated to be susceptible. Haemophilus influenzae meningitis as adjunctive therapy with parenteral streptomycin Meningococcal meningitis prophylaxis. Acute otitis media due to Haemophilus influenzae when used concomitantly with adequate doses of penicillin or erythromycin (see appropriate labeling for prescribing information). Trachoma, inclusion conjunctivitis, nocardiosis, chancroid, toxoplasmosis as adjunctive therapy with pyrimethamine. Malaria due to chloroquine-resistant strains of Plasmodium falciparum, when used as adjunctive therapy. Currently, the increasing frequency of resistant organisms is a limitation of the usefulness of antibacterial agents including the sulfonamides, especially in the treatment of chronic and recurrent urinary tract infections.
Sulfisoxazole is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with action against most gram-positive and many gram-negative organisms. Many strains of an individual species may be resistant to this drugf. Sulfonamides inhibit the multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Absorption
Readily absorbed from the gastrointestinal tract. In a pharmacokinetic study, the adjustments for variable renal clearances between oral and intravenous administration and using the unbound plasma concentrations, the bioavailability for an oral dose of sulfisoxazole was found to be 0.95 +/- 0.04.
Route of Elimination
The mean urinary excretion recovery following oral administration of sulfisoxazole is 97% within 48 hours, of which 52% is the parent drug, and the remaining as the N4-acetylated metabolite. It is excreted in human milk. Sulfisoxazole and its acetylated metabolites are excreted primarily by the kidneys through glomerular filtration.
Volume of Distribution
The sulfonamides are widely distributed throughout all body tissues. It readily crosses the placental barrier and enters into fetal circulation and also crosses the blood-brain barrier. In healthy subjects, cerebrospinal fluid concentrations of sulfisoxazole vary; in patients with meningitis, however, concentrations of free drug in cerebrospinal fluid as high as 94 mcg/mL have been reported. In a pharmacokinetic study, the apparent volume of distribution for total drug was 10.9 +/- 2.0 liters and 136 +/- 36 liters for the unbound drug, indicating that sulfisoxazole is primarily distributed extracellularly.
Clearance
The clearance of sulfisoxazole is 18.7 +/- 3.9 ml/min for total drug and 232 +/- 64 ml/min for the unbound drug.
Sulfisoxazole acetyl is a prodrug of _sulfisoxazole_. The acetyl group is added to make the drug poorly water-soluble and is hydrolyzed in vivo to the active drug,. N1-acetyl sulfisoxazole is metabolized to sulfisoxazole by digestive enzymes in the gastrointestinal tract and is absorbed as sulfisoxazole. This enzymatic splitting is thought to be responsible for slower absorption and lower peak blood concentrations are achieved after administration of an equal oral dose of sulfisoxazole. With sustained administration of acetyl sulfisoxazole, blood concentrations approximate those of sulfisoxazole. Following a single 4 gram dose of acetyl sulfisoxazole to healthy volunteers, maximum plasma concentrations of sulfisoxazole ranged from 122 to 282 mcg/mL (mean, 181 mcg/mL) for the pediatric suspension and occurred between 2 and 6 hours postadministration of sulfisoxazole, in a pharmacokinetic study.
The serum half-life is 10 -12 hours.
Sulfisoxazole is a competitive inhibitor of the enzyme dihydropteroate synthetase. It inhibits bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
