


1. Cra 024781
2. Cra-024781
3. Cra024781
4. Pci 24781
5. Pci-24781
6. Pci24781
1. 783355-60-2
2. Pci-24781
3. Pci 24781
4. Cra-024781
5. Cra 024781
6. Cra-02478
7. Pci24781
8. Cra 24781
9. Abexinostat [usan]
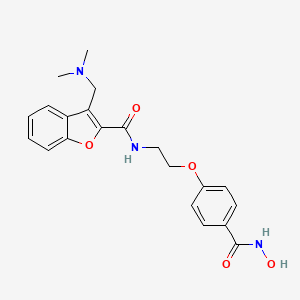
10. 3-((dimethylamino)methyl)-n-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)benzofuran-2-carboxamide
11. Abexinostat (pci-24781)
12. Pci-24781 (abexinostat)
13. Pci-24781 (cra-024781)
14. S78454
15. Iyo470654u
16. Pzp115891
17. 3-((dimethylamino)methyl)-n-(2-(4-(hydroxycarbamoyl)-phenoxy)ethyl)benzofuran-2-carboxamide
18. Abexinostat (usan)
19. Pzp-115891
20. 3-(dimethylaminomethyl)-n-[2-[4-(hydroxycarbamoyl)phenoxy]ethyl]-1-benzofuran-2-carboxamide
21. 3-[(dimethylamino)methyl]-n-[2-[4-(hydroxycarbamoyl)phenoxy]ethyl]-1-benzofuran-2-carboxamide
22. S-78454
23. 3-[(dimethylamino)methyl]-n-[2-[4-[(hydroxyamino)carbonyl]phenoxy]ethyl]-2-benzofurancarboxamide
24. 3-[(dimethylamino)methyl]-n-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-1-benzofuran-2-carboxamide
25. 2-benzofurancarboxamide, 3-((dimethylamino)methyl)-n-(2-(4-((hydroxyamino)carbonyl)phenoxy)ethyl)-
26. Abexinostat [usan:inn]
27. Unii-iyo470654u
28. 3-((dimethylamino)methyl)-n-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)-1-benzofuran-2-carboxamide
29. Cra024781
30. Abexinostat [inn]
31. Abexinostat(pci-24781)
32. Abexinostat [who-dd]
33. Mls006011097
34. Schembl444280
35. Gtpl8366
36. Chembl2103863
37. Bdbm24622
38. Chebi:92223
39. Dtxsid30229005
40. Bcpp000128
41. Hms3654a19
42. Ex-a2123
43. Zinc6716700
44. Cra-24781
45. Fd5037
46. Mfcd10565969
47. Nsc764136
48. Akos025149423
49. Bcp9001054
50. Ccg-264814
51. Cs-0478
52. Db12565
53. Nsc-764136
54. Sb16670
55. Ncgc00346486-01
56. Ncgc00346486-05
57. Ac-26866
58. Bs-18153
59. Hy-10990
60. Smr004702885
61. Db-075461
62. Pci-24781,cra-024781
63. Ft-0673532
64. Sw218266-2
65. Ec-000.2348
66. D10060
67. J-511438
68. Q4667249
69. Brd-k12867552-001-01-3
| Molecular Weight | 397.4 g/mol |
|---|---|
| Molecular Formula | C21H23N3O5 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 397.16377084 g/mol |
| Monoisotopic Mass | 397.16377084 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Histone Deacetylase Inhibitors
Compounds that inhibit HISTONE DEACETYLASES. This class of drugs may influence gene expression by increasing the level of acetylated HISTONES in specific CHROMATIN domains. (See all compounds classified as Histone Deacetylase Inhibitors.)
Abexinostat is a novel histone deacetylase (HDAC) inhibitor. HDAC inhibitors target HDAC enzymes and inhibit the proliferation of cancer cells and induce cancer cell death, or apoptosis. Histone deacetylation is carried out by a family of related HDAC enzymes. Inhibition of these enzymes causes changes to chromatin structure and to gene expression patterns, which results in the inhibition of proliferation of cancer cells, and induction of apoptosis.
