

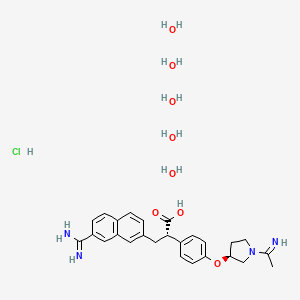

1. (2s)-2-(4-(((3s)-1-acetimidoyl-3-pyrrolidinyl)oxy)phenyl)-3-(7-amidino-2-naphtyl)propanoic Acid
2. Dx 9065
3. Dx 9065a
4. Dx-9065a
1. Dx-9065a
2. 155204-81-2
3. Qxqvepevi2
4. Dx-9065a Hcl Hydrate
5. 155204-81-2 (hcl, Hydrate)
6. X-9065a
7. Dx 9065a
8. (s)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-(((s)-1-(1-iminoethyl)pyrrolidin-3-yl)oxy)phenyl)propanoic Acid Hydrochloride Pentahydrate
9. Dx-9065 Hydrochloride Pentahydrate
10. Unii-qxqvepevi2
11. (s-(r*,r*))-7-(aminoiminomethyl)-alpha-(4-((1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-2-naphthalenepropanoic Acid Monohydrochloride, Pentahydrate
12. Dtxsid60935162
13. (2s)-2-(4-(((3s)-1-acetimidoyl-3-pyrrolidinyl)oxy)phenyl)-3-(7-amidino-2-naphtyl)propanoic Acid
14. 2-naphthalenepropanoic Acid, 7-(aminoiminomethyl)-alpha-(4-((1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-, Monohydrochloride, Pentahydrate, (s-(r*,r*))-
15. (+)-(2s)-2-[4-[[(3s)-1-acetimidoyl-3-pyrrolidi-nyl]oxy]phenyl]-3-[7-amidino-2-naphthyl] Propanoic Acid Hydrochloride Pentahydrate
16. (.alpha.s)-7-(aminoiminomethyl)-.alpha.-(4-(((3s)-1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-2-naphthalenepropanoic Acid Hydrochloride Hydrate (1:1:5)
17. (2s)-3-(7-carbamimidoylnaphthalen-2-yl)-2-[4-[(3s)-1-ethanimidoylpyrrolidin-3-yl]oxyphenyl]propanoic Acid;pentahydrate;hydrochloride
18. 2-naphthalenepropanoic Acid, 7-(aminoiminomethyl)-.alpha.-(4-(((3s)-1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-, Hydrochloride, Hydrate (1:1:5), (.alpha.s)-
19. 2-naphthalenepropanoic Acid, 7-(aminoiminomethyl)-.alpha.-(4-(((3s)-1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-, Monohydrochloride, Pentahydrate, (.alpha.s)-
20. 2-naphthalenepropanoic Acid, 7-(aminoiminomethyl)-.alpha.-(4-((1-(1-iminoethyl)-3-pyrrolidinyl)oxy)phenyl)-, Monohydrochloride, Pentahydrate, (s-(r*,r*))-
21. 2-naphthalenepropanoic Acid,7-(aminoiminomethyl)-a-[4-[[(3s)-1-(1-iminoethyl)-3-pyrrolidinyl]oxy]phenyl]-,hydrochloride,hydrate (1:1:5),(as)-
22. 3-(7-carbamimidoylnaphthalen-2-yl)-2-{4-[(1-ethanimidoylpyrrolidin-3-yl)oxy]phenyl}propanoic Acid--hydrogen Chloride--water (1/1/5)
| Molecular Weight | 571.1 g/mol |
|---|---|
| Molecular Formula | C26H39ClN4O8 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 8 |
| Exact Mass | 570.2456419 g/mol |
| Monoisotopic Mass | 570.2456419 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 720 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 7 |
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Serine Proteinase Inhibitors
Exogenous or endogenous compounds which inhibit SERINE ENDOPEPTIDASES. (See all compounds classified as Serine Proteinase Inhibitors.)
