

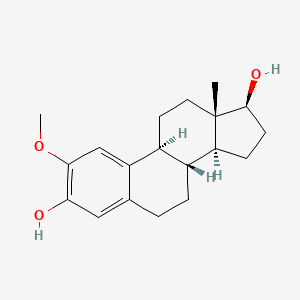

1. (17 Beta)-2-methoxyestra-1,3,5(10)-triene-3,17-diol
2. 2 Methoxyestradiol
3. 2 Methoxyestradiol 17 Beta
4. 2 Methoxyoestradiol
5. 2-(methyl-11c)methoxyestradiol
6. 2-methoxyestradiol, (17alpha)-isomer
7. 2-methoxyestradiol-17 Beta
8. 2-methoxyoestradiol
9. Panzem
1. 362-07-2
2. Panzem
3. 2-methoxyestradiol-17beta
4. 2-hydroxyestradol 2-methyl Ether
5. 2-me2
6. 2me2
7. 2-meoe2
8. Estradiol, 2-methoxy-
9. 2-methoxyestradiol (2-meoe2)
10. 2-methoxyoestradiol
11. 2-hydroxyestradiol 2-methyl Ether
12. 2-methoxy-17beta-estradiol
13. Nsc-659853
14. (17beta)-2-methoxyestra-1,3,5(10)-triene-3,17-diol
15. 2-methoxyestra-1,3,5(10)-triene-3,17beta-diol
16. 2-methoxy-estradiol
17. 2-methoxy-beta-estradiol
18. Mls000028819
19. 6i2qw73sr5
20. Chembl299613
21. Chebi:28955
22. Estra-1,3,5(10)-triene-3,17-diol, 2-methoxy-, (17beta)-
23. Panzem Ncd
24. 1,3,5(10)-estratrien-2,3,17-beta-triol 2-methyl Ether
25. 2-methoxy-estra-1,3,5(10)-triene-3,17beta-diol
26. Estra-1,3,5(10)-triene-3,17beta-diol, 2-methoxy-
27. (8r,9s,13s,14s,17s)-2-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
28. (8r,9s,13s,14s,17s)-2-methoxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
29. Smr000058478
30. Estra-1,3,5(10)-triene-3,17-diol, 2-methoxy-, (17b)-
31. (17beta)-2-methoxyestra-1(10),2,4-triene-3,17-diol
32. Pulmolar
33. 2-methoxy-.beta.-estradiol
34. Nsc 659853
35. Unii-6i2qw73sr5
36. Ccris 9405
37. 1lhw
38. Mfcd00010489
39. Opera_id_634
40. 2-methoxyestradiol Powder
41. 2-methoxyestradiol, Powder
42. 2-methoxy 17?-estradiol
43. M 6383
44. Schembl8796
45. 2-meo-e2
46. Lopac0_000739
47. Mls001076279
48. Mls006010142
49. Dtxsid3040938
50. Gtpl12058
51. Hms2230l22
52. Hms3262c20
53. Hms3412h22
54. Hms3676h22
55. Bcp02076
56. Zinc3818826
57. 2-methoxyestradiol [who-dd]
58. 2-methoxyoestradiol [mart.]
59. Tox21_500739
60. Bdbm50060957
61. Lmst02010035
62. S1233
63. Akos015901723
64. 2-methoxyestradiol, Analytical Standard
65. Bcp9000109
66. Ccg-204824
67. Cs-0176
68. Db02342
69. Lp00739
70. Sdccgsbi-0050717.p002
71. Smp2_000045
72. Ncgc00094082-03
73. Ncgc00094082-04
74. Ncgc00094082-13
75. Ncgc00261424-01
76. Ac-32086
77. As-19175
78. Bp-25393
79. Hy-12033
80. Eu-0100739
81. M2530
82. C05302
83. C74863
84. Ab00639611-09
85. Ab00639611_10
86. Sr-01000076000
87. 2-methoxy-1,3,5 (10)-estratriene-3,17beta-diol
88. 2-methoxyestra-1,3,5(10)-triene-3,17-diol #
89. Q4596897
90. Sr-01000076000-1
91. Brd-k44408410-001-20-0
92. (17?)-2-methoxyestra-1(10),2,4-triene-3,17-diol
93. (17?)-2-methoxyestra-1,3,5(10)-triene-3,17-diol
94. 17beta-2-methoxyestra-1,3,5(10)-triene-3,17-diol
95. Estra-1,3,5(10)-triene-3,17.beta.-diol, 2-methoxy-
96. Estra-1(10),2,4-triene-3,17-diol, 2-methoxy-, (17beta)-
97. (14beta,17beta)-2-methoxyestra-1(10),2,4-triene-3,17-diol
98. (17.beta.)-2-methoxyestra-1,3,5(10)-triene-3,17-diol
99. B3c40b85-1733-48fe-8418-a39011910d19
100. Estra-1,3,5(10)-triene-3,17-diol, 2-methoxy-, (17.beta.)-
| Molecular Weight | 302.4 g/mol |
|---|---|
| Molecular Formula | C19H26O3 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 302.18819469 g/mol |
| Monoisotopic Mass | 302.18819469 g/mol |
| Topological Polar Surface Area | 49.7 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 425 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of breast cancer and inflammatory diseases such as rheumatoid arthritis.
2-Methoxyestradiol belongs to the family of drugs called angiogenesis inhibitors. It also acts as a vasodilator.
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
In vivo metabolism, assessed using 24-h collections of urine from cancer patients treated with 2ME2 revealed that <0.01% of the total administered dose of 2ME2 is excreted unchanged in urine and about 1% excreted as glucuronides. Collectively, this suggests that glucuronidation and subsequent urinary excretion are elimination pathways for 2ME2.
2-O-methoxyestradiol has known human metabolites that include 2-Methoxy-estradiol-17beta 3-glucuronide.
2-O-methoxyestradiol is a known human metabolite of 2-hydroxyestradiol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
2-Methoxyestradiol is an angiogenesis inhibitor, and has been shown to attack both tumor cells and their blood supply in preclinical testing. 2-methoxyestradiol is a naturally occurring estrogen metabolite but has no undesired estrogenic activity.
