
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
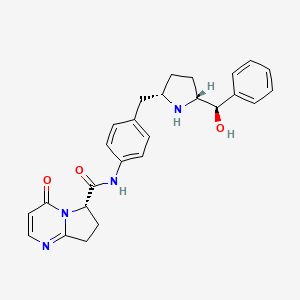

1. Mk-4618
2. N-(4-((5-(hydroxy(phenyl)methyl)pyrrolidin-2-yl)methyl)phenyl)-4-oxo-4,6,7,8-tetrahydropyrrolo(1,2-a)pyrimidine-6-carboxamide
1. 1190389-15-1
2. Krp-114v
3. Gemtesa
4. Mk-4618
5. (s)-n-(4-(((2s,5r)-5-((r)-hydroxy(phenyl)methyl)pyrrolidin-2-yl)methyl)phenyl)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide
6. M5tse03w5u
7. C26h28n4o3
8. Mk4618
9. (6s)-n-(4-(((2s,5r)-5-((r)-hydroxyphenylmethyl)pyrrolidin-2-yl)methyl)phenyl)-4-oxo-4,6,7,8-tetrahydropyrrolo(1,2-a)pyrimidine-6-carboxamide
10. Pyrrolo(1,2-a)pyrimidine-6-carboxamide, 4,6,7,8-tetrahydro-n-(4-(((2s,5r)-5-((r)-hydroxyphenylmethyl)-2-pyrrolidinyl)methyl)phenyl)-4-oxo-, (6s)-
11. Beova
12. Pyrrolo[1,2-a]pyrimidine-6-carboxamide, 4,6,7,8-tetrahydro-n-[4-[[(2s,5r)-5-[(r)-hydroxyphenylmethyl]-2-pyrrolidinyl]methyl]phenyl]-4-oxo-, (6s)-
13. Vibegron [usan]
14. Vibegron [usan:inn]
15. Unii-m5tse03w5u
16. Vibegronum
17. Beova (tn)
18. Mk 4618
19. Vibegron (jan/usan)
20. Vibegron [inn]
21. Vibegron [jan]
22. Vibegron [who-dd]
23. Vibegron [orange Book]
24. Chembl2107826
25. Schembl11985457
26. Gtpl10100
27. Dtxsid40152299
28. Chebi:142418
29. Ex-a3390
30. Bdbm50146154
31. Mfcd28502057
32. At23148
33. Compound 7 [pmid: 26709102]
34. Db14895
35. Hy-19933
36. Cs-0016926
37. D10433
38. A903957
39. Q27283524
40. (6s)-4,6,7,8-tetrahydro-n-[4-[[(2s,5r)-5-[(r)-hydroxyphenylmethyl]-2-pyrrolidinyl]methyl]phenyl]-4-oxo-pyrrolo[1,2-a]pyrimidine-6-carboxamide
41. (6s)-4,6,7,8-tetrahydro-n-[4-[[(2s,5r)-5-[(r)-hydroxyphenylmethyl]-2-pyrrolidinyl]methyl]phenyl]-4-oxopyrrolo[1,2-a]pyrimidine-6-carboxamide
42. (6s)-n-(4-(((2s,5r)-5-((r)-hydroxy(phenyl)methyl)pyrrolidin-2-yl(methyl)phenyl)-4-oxo-4,6,7,8-tetrahydropyrrolo(1,2-a)pyrimidine-
43. (6s)-n-[4-({(2s,5r)-5-[(r)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide
44. (6s)-n-[4-[[(2s,5r)-5-[(r)-hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl]phenyl]-4-oxo-7,8-dihydro-6h-pyrrolo[1,2-a]pyrimidine-6-carboxamide
| Molecular Weight | 444.5 g/mol |
|---|---|
| Molecular Formula | C26H28N4O3 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 444.21614077 g/mol |
| Monoisotopic Mass | 444.21614077 g/mol |
| Topological Polar Surface Area | 94 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 782 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vibegron is indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults.
Vibegron selectivity for beta-3 adrenergic receptors is >9000 times higher than for 1AR or 2AR. Vibegron improves clinical symptoms of overactive bladder by increasing bladder capacity without affecting bladder contraction. It significantly increases the functional bladder volume in a dose-dependent manner, which results in prolongation of the interval between voids. In clinical studies, vibegron inhibited detrusor bladder contractions in a concentration-dependent manner, reduced voiding pressure, and increased bladder compliance. In Japanese clinical studies comprising patients with overactive bladder, vibegron significantly improved the frequency of micturition, urgency, and urgency incontinence episodes.
Absorption
The mean Tmax is 1-3 hours. Steady-state concentrations are achieved within 7 days of once-daily dosing.
Route of Elimination
In a radiolabeled drug study, approximately 59% of the radiolabeled dose was recovered in feces, in which 54% of that amount was in the unchanged parent drug form. About 20% of the radioactivity was recovered in urine, in which 19% of the amount was in the unchanged form.
Volume of Distribution
The mean apparent volume of distribution is 6304 L. The average blood-to-plasma concentration ratio is 0.9. According to tissue distribution studies in animals, vibegron does not penetrate the blood-brain barrier, suggesting limited potential for CNS toxicity in humans.
Clearance
There is limited information on the clearance rate of vibegron.
In vitro, CYP3A4 is the main enzyme responsible for the metabolism of vibegron, which plays a minor role in the elimination of vibegron. Two predominant metabolic pathways are oxidation and glucuronidation to form two oxidative metabolites and three glucuronide metabolites. Metabolites have not been fully characterized.
The terminal plasma half-life ranges from 60 to 70 hours. The effective half-life is 30.8 hours.
Overactive bladder is characterized by symptoms of urge urinary incontinence, urgency, and urinary frequency. Bladder filling and emptying are regulated by the coordinated communication between sympathetic and parasympathetic systems. Bladder filling occurs via parasympathetic inhibition and the sympathetic hypogastric nerve releasing norepinephrine, which acts on beta-adrenergic receptors responsible for mediating detrusor muscle relaxation. Symptoms of overactive bladder are thought to be caused by the deterioration of the sensory connections between the bladder, spinal cord and brain, leading to changes in the lower urinary tract and abnormal bladder sensations of the urge to void at small bladder volumes. Beta-3 adrenergic receptors (3ARs) are expressed in the kidneys and lower urinary tract, including ureters, urethra, prostate, and bladder. Vibegron is a selective agonist at 3AR. One vibegron binds to the receptor, 3AR is stimulated and undergoes a conformation change and activates adenylyl cyclases (AC), which promotes the formation of cyclic adenosine monophosphate (cAMP). Increased intracellular cAMP concentration leads to the activation of cAMP-dependent protein kinase A (PKA), which subsequently phosphorylates myosin light chains that are responsible for inhibiting the interaction of actin with myosin dependent on calcium calmodulin complex. In clinical trials, vibegron increased cAMP levels in a dose-proportional manner. There is evidence that 3AR agonists may also work via sensory mechanisms without directly affecting detrusor muscle motor function.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2024-07-31
Pay. Date : 2024-06-13
DMF Number : 39538
Submission : 2024-03-21
Status : Active
Type : II

Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2024-11-07
Registration Number : Su434-69-ND
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi – 391 450, Dist : Vadodara, Gujarat State, India

 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-11-12
Pay. Date : 2024-09-24
DMF Number : 40333
Submission : 2024-09-27
Status : Active
Type : II

Registrant Name : Lee Sung International Co., Ltd.
Registration Date : 2025-10-10
Registration Number : Su21-34-ND
Manufacturer Name : Honour Lab Limited Unit-III
Manufacturer Address : Plot No.4, Hetero Infrastructure SEZ Ltd, N.Narasapuram Village, Nakkapalli Mandal, Anakapalli District - 531 081, Andhra Pradesh, India
 Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-08-16
Pay. Date : 2024-07-19
DMF Number : 40068
Submission : 2024-07-14
Status : Active
Type : II

Registrant Name : Masung LS Co., Ltd.
Registration Date : 2025-02-13
Registration Number : Su163-40-ND
Manufacturer Name : Yangzhou Aurisco Pharmaceutical Co., Ltd
Manufacturer Address : No.28, Jian'an Road, High-Tech Industrial Development Zone, Yangzhou city, Jiangsu Province, PR China



GDUFA
DMF Review : Reviewed
Rev. Date : 2024-10-22
Pay. Date : 2024-09-26
DMF Number : 40304
Submission : 2024-09-24
Status : Active
Type : II

NDC Package Code : 50379-0027
Start Marketing Date : 2024-09-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40275
Submission : 2024-08-01
Status : Active
Type : II

NDC Package Code : 75877-0008
Start Marketing Date : 2023-05-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Agerson Bio Co., Ltd.
Registration Date : 2025-03-04
Registration Number : Su6191-1-ND(1)
Manufacturer Name : Porton Pharma Solutions Ltd.
Manufacturer Address : 1 Fine Chemical Zone, Chongqing Chemical Industry Park, Changshou, Chongqing 401221, China



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2024-07-31
Pay. Date : 2024-06-13
DMF Number : 39538
Submission : 2024-03-21
Status : Active
Type : II
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
GDUFA
DMF Review : Complete
Rev. Date : 2024-11-12
Pay. Date : 2024-09-24
DMF Number : 40333
Submission : 2024-09-27
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2024-08-16
Pay. Date : 2024-07-19
DMF Number : 40068
Submission : 2024-07-14
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2024-10-22
Pay. Date : 2024-09-26
DMF Number : 40304
Submission : 2024-09-24
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40275
Submission : 2024-08-01
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2024-11-07
Registration Number : Su434-69-ND
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi – 391 45...
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Registrant Name : Lee Sung International Co., Ltd.
Registration Date : 2025-10-10
Registration Number : Su21-34-ND
Manufacturer Name : Honour Lab Limited Unit-III
Manufacturer Address : Plot No.4, Hetero Infrastructure SEZ Ltd, N.Narasapuram Village, Nakkapalli Mandal, A...
Registrant Name : Masung LS Co., Ltd.
Registration Date : 2025-02-13
Registration Number : Su163-40-ND
Manufacturer Name : Yangzhou Aurisco Pharmaceuti...
Manufacturer Address : No.28, Jian'an Road, High-Tech Industrial Development Zone, Yangzhou city, Jiangsu Pr...

Registrant Name : Agerson Bio Co., Ltd.
Registration Date : 2025-03-04
Registration Number : Su6191-1-ND(1)
Manufacturer Name : Porton Pharma Solutions Ltd.
Manufacturer Address : 1 Fine Chemical Zone, Chongqing Chemical Industry Park, Changshou, Chongqing 401221, ...

Registrant Name : Ikemi Korea Co., Ltd.
Registration Date : 2025-01-23
Registration Number : Su6191-1-ND
Manufacturer Name : Porton Pharma Solutions Ltd.
Manufacturer Address : 1 Fine Chemical Zone, Chongqing Chemical Industry Park, Changshou, Chongqing 401221, ...

Registrant Name : Jeil Pharmaceutical Co., Ltd.
Registration Date : 2022-10-31
Registration Number : Su216-24-ND
Manufacturer Name : Sumitomo Chemicals Co., Ltd....
Manufacturer Address : 3750, Azajuhachicho, Maki, Ampachicho, Ampachi-Gun, Gifu 503-0125, Japan

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 50683-0536
Start Marketing Date : 2021-10-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 50379-0027
Start Marketing Date : 2024-09-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65085-0079
Start Marketing Date : 2024-02-15
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 75877-0008
Start Marketing Date : 2023-05-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

NDC Package Code : 68259-1316
Start Marketing Date : 2024-02-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gemtesa(vibegron) is a beta-3 adrenergic receptor agonist, which is indicated for the treatment of male overactive bladder who are receiving pharmacological therapy for benign prostatic hyperplasia.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Sumitomo Pharma America Announces U.S. FDA Approval Of GEMTESA® for OAB Symptoms
Details : Gemtesa(vibegron) is a beta-3 adrenergic receptor agonist, which is indicated for the treatment of male overactive bladder who are receiving pharmacological therapy for benign prostatic hyperplasia.
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Obgemsa (vibegron) is a beta-3 adrenergic agonist, small molecule, which is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency in adults.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Obgemsa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Pierre Fabre Overactive Bladder Med Wins Approval
Details : Obgemsa (vibegron) is a beta-3 adrenergic agonist, small molecule, which is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency in adults.
Product Name : Obgemsa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gemtesa (vibegron) is a beta-3 adrenergic receptor agonist for treating male overactive bladder (OAB) with benign prostatic hyperplasia.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 13, 2024

Sumitomo Pharma’s Vibegron Supplemental NDA Accepted By FDA
Details : Gemtesa (vibegron) is a beta-3 adrenergic receptor agonist for treating male overactive bladder (OAB) with benign prostatic hyperplasia.
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 13, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Obgemsa (vibegron) is a beta-3 adrenergic agonist indicated for treating overactive bladder symptoms, including urge incontinence and urinary urgency.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Obgemsa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Pierre Fabre Receives Positive CHMP Opinion for OBGEMSA™ in Overactive Bladder
Details : Obgemsa (vibegron) is a beta-3 adrenergic agonist indicated for treating overactive bladder symptoms, including urge incontinence and urinary urgency.
Product Name : Obgemsa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gemtesa (vibegron) is a once-daily beta-3 adrenergic receptor (β3) agonist, is currently under investigation for the treatment of men with overactive bladder (OAB) symptoms receiving pharmacological therapy for benign prostatic hyperplasia.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 11, 2023

Details : Gemtesa (vibegron) is a once-daily beta-3 adrenergic receptor (β3) agonist, is currently under investigation for the treatment of men with overactive bladder (OAB) symptoms receiving pharmacological therapy for benign prostatic hyperplasia.
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KYORIN will grant Sumitomo Pharma the exclusive rights to develop, manufacture, and commercialize the therapeutic agent for OAB called Vibegron in in Taiwan and Other Asian Countries, and Sumitomo Pharma will develop, manufacture, and commercialize the Compound.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Sumitomo
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement March 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Sumitomo
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Details : KYORIN will grant Sumitomo Pharma the exclusive rights to develop, manufacture, and commercialize the therapeutic agent for OAB called Vibegron in in Taiwan and Other Asian Countries, and Sumitomo Pharma will develop, manufacture, and commercialize the C...
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Undisclosed
March 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GEMTESA (vibegron) is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 27, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Urovant Sciences Announces Publication of Pharmacokinetic Data on GEMTESA® (Vibegron 75mg) Admini...
Details : GEMTESA (vibegron) is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults.
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 27, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vibegron is a Other Small Molecule drug candidate, which is currently being evaluated in phase II/ phase III clinical studies for the treatment of Neurogenic Detrusor Overactivity.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Undisclosed
Study Phase: Phase II/ Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Vibegron is a Other Small Molecule drug candidate, which is currently being evaluated in phase II/ phase III clinical studies for the treatment of Neurogenic Detrusor Overactivity.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Urovant Sciences and Pierre Fabre will share responsibility for Gemtesa (vibegron), clinical trials in the pediatric populations in Europe. As part of the transaction, Urovant Sciences will also provide manufacturing services to Pierre Fabre.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Obgemsa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Pierre Fabre
Deal Size: $75.0 million Upfront Cash: Undisclosed
Deal Type: Licensing Agreement July 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Pierre Fabre
Deal Size : $75.0 million
Deal Type : Licensing Agreement
Urovant Sciences and Pierre Fabre Médicament Enter into Exclusive License Agreement to Commercial...
Details : Urovant Sciences and Pierre Fabre will share responsibility for Gemtesa (vibegron), clinical trials in the pediatric populations in Europe. As part of the transaction, Urovant Sciences will also provide manufacturing services to Pierre Fabre.
Product Name : Obgemsa
Product Type : Miscellaneous
Upfront Cash : Undisclosed
July 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
In a subgroup analysis of adults, 65 years old or above with overactive bladder, treatment with GEMTESA (Vibegron) was safe and well-tolerated. Treatment with GEMTESA was associated with sustained reductions from baseline in average daily micturition.
Lead Product(s): Vibegron,Inapplicable
Therapeutic Area: Urology Brand Name: Gemtesa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 15, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vibegron,Inapplicable
Therapeutic Area : Urology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Urovant Sciences® Presents New Data from EMPOWUR Study, Advancing Knowledge of the Treatment of O...
Details : In a subgroup analysis of adults, 65 years old or above with overactive bladder, treatment with GEMTESA (Vibegron) was safe and well-tolerated. Treatment with GEMTESA was associated with sustained reductions from baseline in average daily micturition.
Product Name : Gemtesa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 15, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results](6S)-4,6,7,8-tetrahydro-4-oxo-Pyrrolo[1,2-a]pyriMi...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Accel Pharmtech, LLC is one of the world’s leading pharmaceutical companies that manufactures and provides their clients and partners with cataloged chemicals...

(6S)-4-OXO-4,6,7,8-TETRAHYDRO PYRROLO [1,2-A] PYR...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

(6S)-4,6,7,8-tetrahydro-4-oxo-Pyrrolo[1,2-a]pyriMi...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Hangzhou Hairui Chemical Co., Ltd. is located in the Zhongheng Century Science and Technology Park in Hangzhou, Zhejiang. It is an excellent manufacturer of org...

(1R,2R)-2-Amino-6-(4-nitrophenyl)-1-phenylhex-5-yn...
CAS Number : 1628836-08-7
End Use API : Vibegron
About The Company : JieJie Group Co., Ltd. is a pharmaceutical and chemical enterprise for research and production, it was built in 2009. Our core business is custom synthesis and ...

(6S)-4,6,7,8-tetrahydro-4-oxo-Pyrrolo[1,2-a]pyriMi...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Jinan Shangbo Pharmaceutical Co., Ltd is a high-tech enterprise that specializes in the R&D, production, and sales of pharmaceutical intermediates and fine chem...

(1R,2R)-2-Amino-6-(4-nitrophenyl)-1-phenylhex-5-yn...
CAS Number : 1628836-08-7
End Use API : Vibegron
About The Company : Jinan Shangbo Pharmaceutical Co., Ltd is a high-tech enterprise that specializes in the R&D, production, and sales of pharmaceutical intermediates and fine chem...

(1R,2R)-2-amino-6-(4-nitrophenyl)-1-phenylhex-5- y...
CAS Number : 1628836-08-7
End Use API : Vibegron
About The Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & ope...

(6S)-4,6,7,8-tetrahydro-4- oxo-Pyrrolo[1,2- a]pyri...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & ope...

Sodium(S)-4-oxo-4,6,7,8- tetrahydropyrrolo[1,2- a]...
CAS Number : 1421271-01-3
End Use API : Vibegron
About The Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & ope...

(6S)-4,6,7,8-tetrahydro-4-oxo-Pyrrolo[1,2-a]pyriMi...
CAS Number : 1190392-22-3
End Use API : Vibegron
About The Company : Taizhou Volsen Chemical Co., Ltd. is professional in R&D, producing and marketing Pharmaceutical raw materials, pharmaceutical intermediates in China. VOLSEN co...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Obgemsa
Dosage Form : Film Coated Tablet
Dosage Strength : 75mg
Packaging :
Approval Date : 27/06/2024
Application Number : 20230420000029
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Obgemsa
Dosage Form : Film Coated Tablet
Dosage Strength : 75MG
Packaging :
Approval Date : 2024-07-30
Application Number : 1241822001
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : GEMTESA
Dosage Form : TABLET;ORAL
Dosage Strength : 75MG
Packaging :
Approval Date : 2020-12-23
Application Number : 213006
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : GEMTESA
Dosage Form : TABLET;ORAL
Dosage Strength : 75MG
Approval Date : 2020-12-23
Application Number : 213006
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Obgemsa
Dosage Form : Film Coated Tablet
Dosage Strength : 75mg
Packaging :
Approval Date : 27/06/2024
Application Number : 20230420000029
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Obgemsa
Dosage Form : Film Coated Tablet
Dosage Strength : 75MG
Packaging :
Approval Date : 2024-07-30
Application Number : 1241822001
Regulatory Info : Authorized
Registration Country : Spain

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Main Therapeutic Indication : Urology
Currency : USD
2021 Revenue in Millions : 35
2020 Revenue in Millions : 0
Growth (%) : 100

Main Therapeutic Indication : Urology
Currency : USD
2022 Revenue in Millions : 153
2021 Revenue in Millions : 35
Growth (%) : 332

Main Therapeutic Indication : Urology
Currency : USD
2023 Revenue in Millions : 55
2022 Revenue in Millions : 153
Growth (%) : -59

Main Therapeutic Indication : Urology
Currency : USD
2024 Revenue in Millions : 360
2023 Revenue in Millions : 254
Growth (%) : 42

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
15 Jul 2025
Reply
15 Nov 2023
Reply
02 Jun 2023
Reply
05 May 2023
Reply
27 Mar 2023
Reply
17 May 2022
Reply
27 Oct 2021
Reply
07 Sep 2021
Reply
19 Apr 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
63
PharmaCompass offers a list of Vibegron API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vibegron manufacturer or Vibegron supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vibegron manufacturer or Vibegron supplier.
PharmaCompass also assists you with knowing the Vibegron API Price utilized in the formulation of products. Vibegron API Price is not always fixed or binding as the Vibegron Price is obtained through a variety of data sources. The Vibegron Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vibegron manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vibegron, including repackagers and relabelers. The FDA regulates Vibegron manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vibegron API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vibegron manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vibegron supplier is an individual or a company that provides Vibegron active pharmaceutical ingredient (API) or Vibegron finished formulations upon request. The Vibegron suppliers may include Vibegron API manufacturers, exporters, distributors and traders.
click here to find a list of Vibegron suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Vibegron DMF (Drug Master File) is a document detailing the whole manufacturing process of Vibegron active pharmaceutical ingredient (API) in detail. Different forms of Vibegron DMFs exist exist since differing nations have different regulations, such as Vibegron USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Vibegron DMF submitted to regulatory agencies in the US is known as a USDMF. Vibegron USDMF includes data on Vibegron's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Vibegron USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Vibegron suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Vibegron Drug Master File in Korea (Vibegron KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Vibegron. The MFDS reviews the Vibegron KDMF as part of the drug registration process and uses the information provided in the Vibegron KDMF to evaluate the safety and efficacy of the drug.
After submitting a Vibegron KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Vibegron API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Vibegron suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Vibegron as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Vibegron API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Vibegron as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Vibegron and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Vibegron NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Vibegron suppliers with NDC on PharmaCompass.
Vibegron Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vibegron GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vibegron GMP manufacturer or Vibegron GMP API supplier for your needs.
A Vibegron CoA (Certificate of Analysis) is a formal document that attests to Vibegron's compliance with Vibegron specifications and serves as a tool for batch-level quality control.
Vibegron CoA mostly includes findings from lab analyses of a specific batch. For each Vibegron CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vibegron may be tested according to a variety of international standards, such as European Pharmacopoeia (Vibegron EP), Vibegron JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vibegron USP).

