
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Brexafemme
2. Ibrexafungerp
3. Mk-3118
4. Scy-078
1. Scy-078 Citrate
2. M4nu2sdx3e
3. Ibrexafungerp Citrate [usan]
4. 1965291-08-0
5. Ibrexafungerp Citrate (usan)
6. Brexafemme
7. Unii-m4nu2sdx3e
8. Chembl4298168
9. Glxc-26020
10. Ibrexafungerp Citrate [who-dd]
11. Ibrexafungerp Citrate [orange Book]
12. D11545
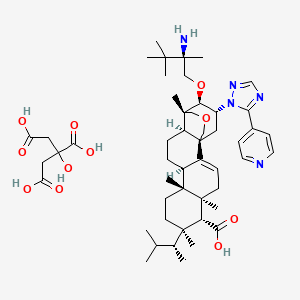
13. (1s,4ar,6as,7r,8r,10ar,10br,12ar,14r,15r)-15-((2r)- 2-amino-2,3,3-trimethylbutoxy)-1,6a,8,10a-tetramethyl-8- ((2r)-3-methylbutan-2-yl)-14-(5-(pyridin-4-yl)-1h-1,2,4- Triazol-1-yl)-1,6,6a,7,8,9,10,10a,10b,11,12,12a-dodecahydro-2h,4h-1,4a-propanophenanthro(1,2-c)pyran-7-carboxylic Acid Compound With 2-hydroxypropane-1,2,3-tricarboxylic Acid (1:1)
14. 4h-1,4a-propano-2h-phenanthro(1,2-c)pyran-7-carboxylic Acid, 15-((2r)-2-amino-2,3,3-trimethylbutoxy)-8-((1r)-1,2-dimethylpropyl)-1,6,6a,7,8,9,10,10a,10b,11,12,12a-dodecahydro-1,6a,8,10a-tetramethyl-14-(5-(4-pyridinyl)-1h-1,2,4-triazol-1-yl)-, (1s,4ar,6as
15. 4h-1,4a-propano-2h-phenanthro(1,2-c)pyran-7-carboxylic Acid, 15-((2r)-2-amino-2,3,3-trimethylbutoxy)-8-((1r)-1,2-dimethylpropyl)-1,6,6a,7,8,9,10,10a,10b,11,12,12a-dodecahydro-1,6a,8,10a-tetramethyl-14-(5-(4-pyridinyl)-1h-1,2,4-triazol-1-yl)-, (1s,4ar,6as,7r,8r,10ar,10br,12ar,14r,15r)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
| Molecular Weight | 922.2 g/mol |
|---|---|
| Molecular Formula | C50H75N5O11 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 14 |
| Exact Mass | 921.54630823 g/mol |
| Monoisotopic Mass | 921.54630823 g/mol |
| Topological Polar Surface Area | 258 Ų |
| Heavy Atom Count | 66 |
| Formal Charge | 0 |
| Complexity | 1650 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
About the Company : Omgene Life Sciences Pvt. Ltd. is an R&D-driven biopharmaceutical company specializing in biopharmaceuticals, peptides, semi-synthetic, and synthetic actives. As a vertically integ...
About the Company : BrightGene Bio-Medical Technology Co., Ltd. is a research driven biopharmaceutical company that engages in the R&D and manufacturing of innovative medicine as well as special gener...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the licensing deal for Ibrexafungerp Citrate, the agreement aims to advance treatment for vulvovaginal candidiasis by targeting 1,3-beta-glucan synthase.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $593.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement November 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : GSK
Deal Size : $593.0 million
Deal Type : Licensing Agreement
Scynexis Transfers BREXAFEMME® NDA to GSK
Details : Through the licensing deal for Ibrexafungerp Citrate, the agreement aims to advance treatment for vulvovaginal candidiasis by targeting 1,3-beta-glucan synthase.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
November 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the licensing deal for Ibrexafungerp Citrate, the agreement aims to advance treatment for invasive candidiasis by targeting 1,3-beta-glucan synthase.
Lead Product(s): Ibrexafungerp Citrate
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $593.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement October 15, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : GSK
Deal Size : $593.0 million
Deal Type : Licensing Agreement
Scynexis Secures $22M Payout from GSK After Trial Dispute
Details : Through the licensing deal for Ibrexafungerp Citrate, the agreement aims to advance treatment for invasive candidiasis by targeting 1,3-beta-glucan synthase.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
October 15, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ibrexafungerp is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Candidiasis, Vulvovaginal.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 01, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Pharmacokinetic Study in Healthy Lactating Women Exposed to Ibrexafungerp
Details : Ibrexafungerp is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Candidiasis, Vulvovaginal.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 01, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, GSK has the rights to develop GSK5458448/SCY-078 (ibrexafungerp), a novel oral glucan synthase inhibitor and commercialize Brexafemme in all countries except the greater China.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Phase II/ Phase IIIProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $593.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement July 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : GSK
Deal Size : $593.0 million
Deal Type : Licensing Agreement
SCYNEXIS to Receive $10M from GSK for Completed FURI, CARES and NATURE Study Reports
Details : Under the agreement, GSK has the rights to develop GSK5458448/SCY-078 (ibrexafungerp), a novel oral glucan synthase inhibitor and commercialize Brexafemme in all countries except the greater China.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
July 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GSK acquired the rights to develop and commercialize GSK5458448 (ibrexafungerp), a glucan synthase inhibitor, and market Brexafemme for antifungal indications outside China.
Lead Product(s): Ibrexafungerp Citrate,Caspofungin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $401.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Caspofungin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : GSK
Deal Size : $401.0 million
Deal Type : Licensing Agreement
GSK Amends Deal with Scynexis Amid Brexafemme Manufacturing Woes
Details : GSK acquired the rights to develop and commercialize GSK5458448 (ibrexafungerp), a glucan synthase inhibitor, and market Brexafemme for antifungal indications outside China.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Brexafemme (ibrexafungerp), a novel glucan synthase inhibitor, approved for vulvovaginal candidiasis and RVVC, is being develop in phase 3 trials for the potential treatment of invasive candidiasis.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Hansoh Pharma
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 20, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Hansoh Pharma
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Brexafemme (ibrexafungerp), a novel glucan synthase inhibitor, approved for vulvovaginal candidiasis and RVVC, is being develop in phase 3 trials for the potential treatment of invasive candidiasis.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 20, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement GSK gains right to commercialize Brexafemme (ibrexafungerp), a novel glucan synthase inhibitor, for vulvovaginal candidiasis and RVVC while continuing to develop ibrexafungerp, in phase 3 trials for the potential treatment of invasive candidiasis.
Lead Product(s): Ibrexafungerp Citrate,Caspofungin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $593.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement June 21, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Caspofungin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : GSK
Deal Size : $593.0 million
Deal Type : Licensing Agreement
Details : Under the agreement GSK gains right to commercialize Brexafemme (ibrexafungerp), a novel glucan synthase inhibitor, for vulvovaginal candidiasis and RVVC while continuing to develop ibrexafungerp, in phase 3 trials for the potential treatment of invasive...
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
June 21, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, GSK has the rights to develop ibrexafungerp, a novel oral glucan synthase inhibitor with a broad spectrum of activity and commercialize Brexafemme (ibrexafungerp tablets) in all countries except the greater China region.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: $593.0 million Upfront Cash: $90.0 million
Deal Type: Licensing Agreement May 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : GSK
Deal Size : $593.0 million
Deal Type : Licensing Agreement
SCYNEXIS Reports Closing of Exclusive License Agreement with GSK for BREXAFEMME® (Ibrexafungerp T...
Details : Under the agreement, GSK has the rights to develop ibrexafungerp, a novel oral glucan synthase inhibitor with a broad spectrum of activity and commercialize Brexafemme (ibrexafungerp tablets) in all countries except the greater China region.
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : $90.0 million
May 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, GSK gains rights to commercialise Brexafemme, a first-in-class antifungal for the treatment of VVC and for reduction in the incidence of RVVC while continuing to develop ibrexafungerp, which is in phase III trials for the potential treatment of IC.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Brexafemme
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: GSK
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement March 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : GSK
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Details : Under the agreement, GSK gains rights to commercialise Brexafemme, a first-in-class antifungal for the treatment of VVC and for reduction in the incidence of RVVC while continuing to develop ibrexafungerp, which is in phase III trials for the potential t...
Product Name : Brexafemme
Product Type : Miscellaneous
Upfront Cash : Undisclosed
March 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ibrexafungerp is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Mycoses.
Lead Product(s): Ibrexafungerp Citrate,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ibrexafungerp Citrate,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
ADME Study of [14^C]-Ibrexafungerp in Healthy Male Subjects
Details : Ibrexafungerp is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Mycoses.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 29, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : BREXAFEMME
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 150MG BASE
Packaging :
Approval Date : 2021-06-01
Application Number : 214900
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2039-06-10
US Patent Number : 11534433
Drug Substance Claim :
Drug Product Claim :
Application Number : 214900
Patent Use Code : U-3159
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2039-06-10

Patent Expiration Date : 2039-06-10
US Patent Number : 11534433
Drug Substance Claim :
Drug Product Claim :
Application Number : 214900
Patent Use Code : U-3508
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2039-06-10

Patent Expiration Date : 2030-08-28
US Patent Number : 8188085
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 214900
Patent Use Code : U-3159
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-08-28

Patent Expiration Date : 2035-01-19
US Patent Number : 10370406
Drug Substance Claim :
Drug Product Claim :
Application Number : 214900
Patent Use Code : U-3159
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-01-19

Patent Expiration Date : 2035-01-19
US Patent Number : 10927142
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 214900
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-01-19

Patent Expiration Date : 2035-01-19
US Patent Number : 10174074
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 214900
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-01-19

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
48
PharmaCompass offers a list of Ibrexafungerp Citrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ibrexafungerp Citrate manufacturer or Ibrexafungerp Citrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ibrexafungerp Citrate manufacturer or Ibrexafungerp Citrate supplier.
PharmaCompass also assists you with knowing the Ibrexafungerp Citrate API Price utilized in the formulation of products. Ibrexafungerp Citrate API Price is not always fixed or binding as the Ibrexafungerp Citrate Price is obtained through a variety of data sources. The Ibrexafungerp Citrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ibrexafungerp Citrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ibrexafungerp Citrate, including repackagers and relabelers. The FDA regulates Ibrexafungerp Citrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ibrexafungerp Citrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ibrexafungerp Citrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ibrexafungerp Citrate supplier is an individual or a company that provides Ibrexafungerp Citrate active pharmaceutical ingredient (API) or Ibrexafungerp Citrate finished formulations upon request. The Ibrexafungerp Citrate suppliers may include Ibrexafungerp Citrate API manufacturers, exporters, distributors and traders.
click here to find a list of Ibrexafungerp Citrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ibrexafungerp Citrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ibrexafungerp Citrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ibrexafungerp Citrate GMP manufacturer or Ibrexafungerp Citrate GMP API supplier for your needs.
A Ibrexafungerp Citrate CoA (Certificate of Analysis) is a formal document that attests to Ibrexafungerp Citrate's compliance with Ibrexafungerp Citrate specifications and serves as a tool for batch-level quality control.
Ibrexafungerp Citrate CoA mostly includes findings from lab analyses of a specific batch. For each Ibrexafungerp Citrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ibrexafungerp Citrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Ibrexafungerp Citrate EP), Ibrexafungerp Citrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ibrexafungerp Citrate USP).

