
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
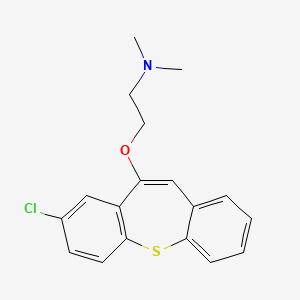

1. 2-chloro-11-(2-dimethylaminoethoxy)dibenzo(b,f)thiepine
2. Nipolept
3. Zoleptil
1. 26615-21-4
2. Nipolept
3. Lodopin
4. Zoleptil
5. Zotepina
6. Zotepinum
7. Setous
8. Zotepin
9. 2-chloro-11-(2-(dimethylamino)ethoxy)dibenzo(b,f)thiepin
10. 2-chlor-11-(2-dimethylaminoaethoxy)-dibenzo(b,f)-thiepin
11. Ethanamine, 2-[(8-chlorodibenzo[b,f]thiepin-10-yl)oxy]-n,n-dimethyl-
12. U29o83jazw
13. Chembl285802
14. Chebi:32316
15. 2-(3-chlorobenzo[b][1]benzothiepin-5-yl)oxy-n,n-dimethylethanamine
16. 2-[(8-chlorodibenzo[b,f]thiepin-10-yl)oxy]-n,n-dimethylethanamine
17. Ncgc00182081-02
18. 2-((8-chlorodibenzo(b,f)thiepin-10-yl)oxy)-n,n-dimethylethanamine
19. 2-((8-chlorodibenzo(b,f)thiepin-10-yl)oxy)-n,n-dimethylethylamine
20. Dsstox_cid_3756
21. Compound-4
22. Dsstox_rid_77187
23. Dsstox_gsid_23756
24. Lodopin; Losizopilon; Nipolept; Setous; Zoleptil;
25. Zotepina [spanish]
26. Zotepinum [inn-latin]
27. Zotepina [inn-spanish]
28. 2-(3-chloranylbenzo[b][1]benzothiepin-5-yl)oxy-n,n-dimethyl-ethanamine
29. Ethanamine, 2-((8-chlorodibenzo(b,f)thiepin-10-yl)oxy)-n,n-dimethyl-
30. Cas-26615-21-4
31. Unii-u29o83jazw
32. Zotepine [inn:ban:jan]
33. Brn 1435710
34. Engramon
35. Majorpin
36. Setous (tn)
37. 2-chloro-11-(2-dimethylaminoethoxy)-dibenzo(b,f)thiepine
38. Zotepine (jan/inn)
39. Zotepine [inn]
40. Zotepine [jan]
41. Zotepine [mi]
42. 2-chlor-11-(2-dimethylaminoaethoxy)-dibenzo(b,f)-thiepin [german]
43. Zotepine [mart.]
44. Zotepine [who-dd]
45. Gtpl103
46. 5-17-04-00588 (beilstein Handbook Reference)
47. Schembl114409
48. Zinc2264
49. Dtxsid9023756
50. Bdbm35255
51. Hdozvruncmbhfh-uhfffaoysa-
52. Hms3713e06
53. Bcp06661
54. Tox21_113135
55. Dibenzo(b,f)thiepin, 2-chloro-11-(2-(dimethylamino)ethoxy)-
56. Akos005066198
57. Tox21_113135_1
58. Ccg-220422
59. Db09225
60. Fr-1314
61. Ncgc00182081-03
62. Ncgc00182081-04
63. As-12557
64. Hy-103093
65. B7563
66. Cs-0024101
67. Ft-0655460
68. D01321
69. 615z214
70. A818524
71. L000902
72. Q226905
73. Sr-01000872710
74. Sr-01000872710-1
75. 2-(8-chlorobenzo[b][1]benzothiepin-6-yl)oxy-n,n-dimethylethanamine
76. 2-((3-chloro-5l4-dibenzo[b,f]thiepin-5-yl)oxy)-n,n-dimethylethan-1-amine
77. 2-[(8-chlorodibenzo(z)[b,f]thiepin- 10-yl)oxy]-n,n-dimethylethanamine
78. 2-[(8-chlorodibenzo(z)[b,f]thiepin-10-yl)oxy]-n,n-dimethylethanamine
79. 1200591-33-8
80. Zot
| Molecular Weight | 331.9 g/mol |
|---|---|
| Molecular Formula | C18H18ClNOS |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 331.0797631 g/mol |
| Monoisotopic Mass | 331.0797631 g/mol |
| Topological Polar Surface Area | 37.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 401 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Zotepine, like other atypical antipsychotics, is considered as the first-line treatment in newly diagnosed schizophrenia. It is usually thought to be an option of choice for managing acute schizophrenic episodes when discussion with the patient is not possible. Zotepine, as an atypical antipsychotic, is used in patients who are suffering unacceptable side effects from conventional antipsychotics or in relapse patients that were inadequately controlled. It is important to consider that the indications stated above are related to atypical antipsychotics, that zotepine is not currently FDA, Canada or EMA approved and that studies have not shown any additional benefit when compared with other approved atypical antipsychotics. Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels and behaves. It is usually marked for a loose reality perspective delineated by hallucinations, delusions and thought and movement disorders.
In preclinical studies, zotepine is characterized by the presence of a strong antiserotonergic activity when compared with other neuroleptic drugs. It has also been reported to present elevations in the seizure threshold in the amygdaloid nucleus. When zotepine's effects were analyzed by electroencephalography, it was noted a typical response of a low-potency neuroleptics of the sedative type. Administration of zotepine has shown improvements in numerical movements and complex reaction. These effects tend to be accompanied by increases in pulse rate, increase in prolactin levels and some typical neuroleptic side effects.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AX - Other antipsychotics
N05AX11 - Zotepine
Absorption
Preclinical pharmacokinetic studies have shown a dose-dependent increase in plasma levels with a tmax between 2-4 hours and Cmax from 6.9-19.6 ng/ml when administered in a dose of 25-100 mg of zotepine. The maximum concentration peaks and slow declines thereafter. When administered orally in preclinical studies, zotepine was proven to be absorbed rapidly and almost completely from the gastrointestinal tract.The unchanged drug and metabolites are rapidly distributed to the tissues.
Route of Elimination
Only small amounts of the unchanged zotepine are excreted in the urine and fecal excretion through the bile is the main route of elimination of both the unchanged drug and its metabolites.
Volume of Distribution
The apparent volume of distribution of zotepine is 109 L/kg.
Clearance
The apparent oral clearance of zotepine is 4.6 mg/h.kg.
Zotepine is well metabolized in the body, it actually undergoes extensive first-pass metabolism to the metabolite norzotepine and several inactive metabolites. The main enzymes involved in zotepine metabolism are CYP1A2 and CYP3A4. Some of the main metabolic pathways include N-demethylation and oxygenation of N or S atoms, hydroxylation of the aromatic ring and consecutive conjugation.
Zotepine has known human metabolites that include 2-hydroxy-zotepine, 3-hydroxy-zotepine, Norzotepine, Zotepine -oxide, and Zotepine N-oxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of zotepine is reported to be of 21 hours.
Zotepine is a dopamine antagonist that has a high affinity for D1- and D2-like receptors. It presents a strong antagonism for several serotonin receptors, such as 5-HT2a, 5-HT2c, 5-HT6 and 5-HT7. Zotepine activities are also related to the inhibition of noradrenaline reuptake and serotonergic activity. All these effects allow zotepine to improve the negative and cognitive symptoms of schizophrenia.
Market Place

ABOUT THIS PAGE
94
PharmaCompass offers a list of Zotepine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zotepine manufacturer or Zotepine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zotepine manufacturer or Zotepine supplier.
PharmaCompass also assists you with knowing the Zotepine API Price utilized in the formulation of products. Zotepine API Price is not always fixed or binding as the Zotepine Price is obtained through a variety of data sources. The Zotepine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zotepine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zotepine, including repackagers and relabelers. The FDA regulates Zotepine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zotepine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Zotepine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Zotepine supplier is an individual or a company that provides Zotepine active pharmaceutical ingredient (API) or Zotepine finished formulations upon request. The Zotepine suppliers may include Zotepine API manufacturers, exporters, distributors and traders.
click here to find a list of Zotepine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Zotepine DMF (Drug Master File) is a document detailing the whole manufacturing process of Zotepine active pharmaceutical ingredient (API) in detail. Different forms of Zotepine DMFs exist exist since differing nations have different regulations, such as Zotepine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Zotepine DMF submitted to regulatory agencies in the US is known as a USDMF. Zotepine USDMF includes data on Zotepine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Zotepine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Zotepine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Zotepine Drug Master File in Japan (Zotepine JDMF) empowers Zotepine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Zotepine JDMF during the approval evaluation for pharmaceutical products. At the time of Zotepine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Zotepine suppliers with JDMF on PharmaCompass.
Zotepine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zotepine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zotepine GMP manufacturer or Zotepine GMP API supplier for your needs.
A Zotepine CoA (Certificate of Analysis) is a formal document that attests to Zotepine's compliance with Zotepine specifications and serves as a tool for batch-level quality control.
Zotepine CoA mostly includes findings from lab analyses of a specific batch. For each Zotepine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zotepine may be tested according to a variety of international standards, such as European Pharmacopoeia (Zotepine EP), Zotepine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zotepine USP).

