



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
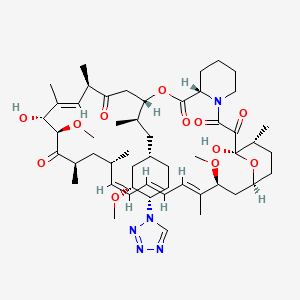

1. Abt 578
2. Abt-578
3. Abt578
4. Zotarilumus
1. Abt-578
2. 221877-54-9
3. Abt578
4. H4gxr80ize
5. Mdt-4107
6. Chembl219410
7. Abt 578
8. A 179578
9. Zotarilumus
10. Unii-h4gxr80ize
11. Zotarolimus [inn]
12. Zotarolimus [usan:inn]
13. Zotarolimus [usan]
14. Zotarolimus (abt-578)
15. Zotarolimus; Abt-578
16. Zotarolimus [mart.]
17. Schembl67389
18. Zotarolimus [who-dd]
19. Gtpl7974
20. Dtxsid50873387
21. Chebi:135897
22. Ex-a2216
23. Bdbm50174276
24. Mfcd09752954
25. S7091
26. Akos037645037
27. Zinc169677012
28. Ccg-270596
29. Cs-5715
30. Ncgc00386351-01
31. Ac-31528
32. As-56346
33. Hy-12424
34. J-014574
35. Q15410168
36. Rapamycin, 42-deoxy-42-(1h-tetrazol-1-yl)-, (42s)-
37. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,32s,35r)-1,18-dihydroxy-19,30-dimethoxy-12-[(2r)-1-[(1s,3r,4s)-3-methoxy-4-(1h-1,2,3,4-tetrazol-1-yl)cyclohexyl]propan-2-yl]-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.0^{4,9}]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
| Molecular Weight | 966.2 g/mol |
|---|---|
| Molecular Formula | C52H79N5O12 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 7 |
| Exact Mass | 965.57252297 g/mol |
| Monoisotopic Mass | 965.57252297 g/mol |
| Topological Polar Surface Area | 219 Ų |
| Heavy Atom Count | 69 |
| Formal Charge | 0 |
| Complexity | 1890 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


