


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
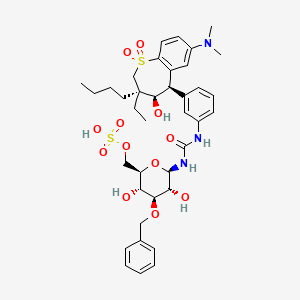

1. Lum002
2. Shp626
1. 1025216-57-2
2. Sar-548304 Free Acid
3. X2jz0451h8
4. N-(3-o-benzyl-6-o-sulfo-beta-d-glucopyranosyl)-n'-{3-[(3s,4r,5r)-3-butyl-7-(dimethylamino)-3-ethyl-4-hydroxy-1,1-dioxo-2,3,4,5-tetrahydro-1h-1lambda6-benzothiepin-5-yl]phenyl}urea
5. Volixibat [usan:inn]
6. Unii-x2jz0451h8
7. Starbld0000127
8. Volixibat [inn]
9. Volixibat [usan]
10. Volixibat [who-dd]
11. Chembl3707222
12. Schembl12196537
13. Dtxsid001098929
14. Db13914
15. Urea, N-(3-((3s,4r,5r)-3-butyl-7-(dimethylamino)-3-ethyl-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl)phenyl)-n'-(3-o-(phenylmethyl)-6-o-sulfo-beta-d-glucopyranosyl)-
16. Hy-101190
17. Cs-0020958
18. Q27293432
19. [(2r,3r,4s,5r,6r)-6-[[3-[(3s,4r,5r)-3-butyl-7-(dimethylamino)-3-ethyl-4-hydroxy-1,1-dioxo-4,5-dihydro-2h-1lambda6-benzothiepin-5-yl]phenyl]carbamoylamino]-3,5-dihydroxy-4-phenylmethoxyoxan-2-yl]methyl Hydrogen Sulfate
20. N-[3-[(3s,4r,5r)-3-butyl-7-(dimethylamino)-3-ethyl-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl]phenyl]-n'-[3-o-(phenylmethyl)-6-o-sulfo-beta-d-glucopyranosyl]urea
21. Urea, N-(3-((3s,4r,5r)-3-butyl-7-(dimethylamino)-3-ethyl-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl)phenyl)-n'-(3-o-(phenylmethyl)-6-o-sulfo-.beta.-d-glucopyranosyl)-
| Molecular Weight | 806.0 g/mol |
|---|---|
| Molecular Formula | C38H51N3O12S2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 14 |
| Exact Mass | 805.29141642 g/mol |
| Monoisotopic Mass | 805.29141642 g/mol |
| Topological Polar Surface Area | 238 Ų |
| Heavy Atom Count | 55 |
| Formal Charge | 0 |
| Complexity | 1450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Volixibat is an investigational drug that has not been approved for use in any conditions.
Lipid Regulating Agents
Substances that alter the metabolism of LIPIDS. (See all compounds classified as Lipid Regulating Agents.)
Volixibat is a selective inhibitor of the apical sodium-dependent bile acid transporter (ASBT), a transmembrane protein primarily expressed by enterocytes of the ileum. ASBT is primarily responsible for the recirculation of bile acids and therefore for hepatic lipid and glucose metabolism. Inhibiting this enzyme results in a decrease of bile acids returning to the liver, which is helpful for the treatment of NASH as abnormal cholesterol metabolism and accumulation of free cholesterol in the liver have been implicated in its pathogenesis.


