
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
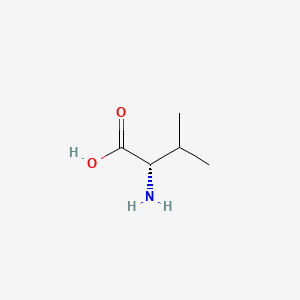

1. L Valine
2. L-valine
1. L-valine
2. 72-18-4
3. (s)-valine
4. H-val-oh
5. (s)-2-amino-3-methylbutanoic Acid
6. (2s)-2-amino-3-methylbutanoic Acid
7. 2-amino-3-methylbutyric Acid
8. (s)-2-amino-3-methylbutyric Acid
9. L-alpha-amino-beta-methylbutyric Acid
10. Valinum [latin]
11. Valina [spanish]
12. Valine (van)
13. Butanoic Acid, 2-amino-3-methyl-
14. Valine [usan:inn]
15. L-valin
16. Valine, L-
17. L(+)-alpha-aminoisovaleric Acid
18. (s)-alpha-amino-beta-methylbutyric Acid
19. 7004-03-7
20. Butanoic Acid, 2-amino-3-methyl-, (s)-
21. 2-amino-3-methylbutyric Acid, (s)-
22. 2-amino-3-methylbutanoic Acid, (s)-
23. Val
24. Nsc 76038
25. (l)-valine
26. 2-amino-3-methylbutanoic Acid (van)
27. Valine (l-valine)
28. Chebi:16414
29. L-(+)-alpha-aminoisovaleric Acid
30. L-2-aminoisovaleric Acid
31. Nsc-76038
32. Valina
33. Hg18b9yrs7
34. L-val
35. Mfcd00064220
36. Valinum
37. (2s)-2-amino-3-methylbutanoate
38. L-2-amino-3-methylbutanoic Acid
39. Alpha-aminoisovaleric Acid
40. Einecs 200-773-6
41. 2-amino-3-methyl-butyric Acid
42. Unii-hg18b9yrs7
43. 2-amino-3-methylbutanoate
44. Racemic Valine
45. L-(+)-a-aminoisovaleric Acid
46. S-valin
47. (s)-alpha-aminoisovaleric Acid
48. L-(+)-.alpha.-aminoisovaleric Acid
49. L-a-amino-b-methylbutyric Acid
50. Hsdb 7800
51. 3h-l-valine
52. (s)-a-amino-b-methylbutyric Acid
53. L-valine;
54. L-valine, Fcc
55. Valine (usp)
56. (+)-valine
57. L-valine,(s)
58. 1t4s
59. L-valine (jp17)
60. L-valine, 99%
61. Valine [vandf]
62. Valine [hsdb]
63. Valine [inci]
64. Valine [usan]
65. (s)-val
66. L-val-4
67. L-val-oh
68. Valine [inn]
69. Valine [who-dd]
70. Valine [ii]
71. Valine [mi]
72. 2-amino-3-methylbutyrate
73. L-valine [fcc]
74. L-valine [jan]
75. Valine [mart.]
76. Bmse000052
77. Bmse000811
78. Bmse000860
79. Ec 200-773-6
80. L-(+)-a-aminoisovalerate
81. L-a-amino-b-methylbutyrate
82. Schembl8516
83. (s)-a-aminoisovaleric Acid
84. L-valine [usp-rs]
85. H-val-2-chlorotrityl Resin
86. (s)-?-aminoisovaleric Acid
87. 2-aminoisovaleric Acid,(s)
88. Chembl43068
89. Valine [ep Monograph]
90. (s)-a-amino-b-methylbutyrate
91. L-(+)-alpha-aminoisovalerate
92. L-iso-c3h7ch(nh2)cooh
93. Valine [usp Monograph]
94. Gtpl4794
95. (s)-2-amino-3-methylbutyrate
96. (s)-2-amino-3-methylbutanoate
97. Dtxsid40883233
98. L-alpha-amino-beta-methylbutyrate
99. (s)-2-amino-3-methyl-butanoate
100. Pharmakon1600-01301009
101. Zinc895099
102. Hy-n0717
103. (s)-alpha-amino-beta-methylbutyrate
104. (s)-2-amino-3-methyl-butyric Acid
105. Bdbm50463208
106. Nsc760111
107. S5628
108. (s)-2-amino-3-methyl-butanoic Acid
109. L-valine, 98.5-101.5%
110. Akos015841564
111. Am82363
112. Ccg-266067
113. Cs-w020706
114. Db00161
115. L-valine, 99%, Natural, Fcc, Fg
116. Nsc-760111
117. Ncgc00344520-01
118. As-12787
119. L-valine, Bioultra, >=99.5% (nt)
120. L-valine, Saj Special Grade, >=98.5%
121. Db-029989
122. L-valine, Reagent Grade, >=98% (hplc)
123. V0014
124. L-valine, Vetec(tm) Reagent Grade, >=98%
125. C00183
126. D00039
127. L-valine, Cell Culture Reagent (h-l-val-oh)
128. Lysine Acetate Impurity D [ep Impurity]
129. M02950
130. V-1800
131. (s)-(+)-2-amino-3-methylbutyric Acid
132. 064v220
133. Q483752
134. Q-100919
135. L-valine, Certified Reference Material, Tracecert(r)
136. F8889-8698
137. Valine, European Pharmacopoeia (ep) Reference Standard
138. Z1250208665
139. 1b39571b-0ae8-4a9a-ae80-4b898d11a981
140. L-valine, Dimer, Meets The Analytical Specifications Of Usp
141. L-valine, United States Pharmacopeia (usp) Reference Standard
142. L-valine, Pharmaceutical Secondary Standard; Certified Reference Material
143. L-valine, From Non-animal Source, Meets Ep, Jp, Usp Testing Specifications, Suitable For Cell Culture, 98.5-101.0%
144. L-valine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 117.15 g/mol |
|---|---|
| Molecular Formula | C5H11NO2 |
| XLogP3 | -2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 117.078978594 g/mol |
| Monoisotopic Mass | 117.078978594 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 90.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A branched-chain essential amino acid that has stimulant activity. It promotes muscle growth and tissue repair. It is a precursor in the penicillin biosynthetic pathway.
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
It is used as a dietary supplement. It is also an ingredient of several preparations that have been promoted for disorders of the liver.
Sweetman SC (ed), Martindale: The Complete Drug Reference. London: Pharmaceutical Press (2009), p.1971.
Branched chain amino acid (BCAA)-enriched protein or amino acid mixtures and, in some cases, BCAA alone, have been used in the treatment of a variety of metabolic disorders. These amino acids have received considerable attention in efforts to reduce brain uptake of aromatic amino acids and to raise low circulating levels of BCAA in patients with chronic liver disease and encephalopathy. They have also been used in parenteral nutrition of patients with sepsis and other abnormalities.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 705, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The aim of this study was to evaluate the compliance of the diet with limited branched-chain amino acids (BCAA) content in long-term observation of patients with maple syrup urine disease (MSUD). The study group consisted of 7 children at age of 1.5-18 years. Nutrition evaluation was based on current diet records from 3-4 days, every 3-4 months. ... Energy and content of most of the nutrients in proposed daily products lists were in agreement with RDI except calcium. Diet analysis at MSUD children revealed insufficient contents of: iron, zinc, copper, vitamin B1, B2, niacin and vitamin C (often below 90% RDI). /Branched chain amino acids/
PMID:17711097 Kowalik A, et al; Rocz Panstw Zakl Hig 58 (1): 95-101.
Assays of the amino acid levels in 5,888 newborns and 20 subjects ranging in age from 1 to 20 years, suspected of metabolic diseases, revealed a case of "maple syrup urine disease" caused by disorders in the intermediate metabolism of valine, whose serum and urinary concentrations were followed up from the first days of life. This patient also showed frequent episodes of hypoglycemia. An early treatment with polyvitamins, minerals and trace elements for 18 months resulted in the partial reactivation of the deficient enzymatic systems and the return to normal of the serum and urinary valine and glucose values. Administration of the same treatment to patients over one year of age ... was much less effective, thus supporting the conclusion that the vitamins and minerals could be useful in the "maple syrup urine disease" only if they were administered immediately after the disease onset ...
PMID:8081313 Mogos, T, et al; Rom J Intern Med 32 (1): 57-61 (1994).
Promotes mental vigor, muscle coordination, and calm emotions. May also be of use in a minority of patients with hepatic encephalopathy and in some with phenylketonuria.
L-valine is a branched-chain essential amino acid (BCAA) that has stimulant activity. It promotes muscle growth and tissue repair. It is a precursor in the penicillin biosynthetic pathway. Valine is one of three branched-chain amino acids (the others are leucine and isoleucine) that enhance energy, increase endurance, and aid in muscle tissue recovery and repair. This group also lowers elevated blood sugar levels and increases growth hormone production. Supplemental valine should always be combined with isoleucine and leucine at a respective milligram ratio of 2:1:2. It is an essential amino acid found in proteins; important for optimal growth in infants and for growth in children and nitrogen balance in adults. The lack of L-valine may influence the growth of body, cause neuropathic obstacle, anaemia. It has wide applications in the field of pharmaceutical and food industry.
Absorption
Absorbed from the small intestine by a sodium-dependent active-transport process.
Blood and tissue concentrations of branched chain amino acids (BCAA) are altered by several disease and abnormal physiological states, including diabetes mellitus, liver dysfunction, starvation, protein-calorie malnutrition, alcoholism, and obesity. These and other conditions sometimes produce drastic alterations in plasma pools of BCAA. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 704, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 596, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 599, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Protein secretion into the intestine continues even under conditions of protein-free feeding, and fecal nitrogen losses (ie, nitrogen lost as bacteria in the feces) may account for 25 percent of the obligatory loss of nitrogen. Under this dietary circumstance, the amino acids secreted into the intestine as components of proteolytic enzymes and from sloughed mucosal cells are the only sources of amino acids for the maintenance of the intestinal bacterial biomass. ... Other routes of loss of intact amino acids are via the urine and through skin and hair loss. These losses are small by comparison with those described above, but nonetheless may have a significant impact on estimates of requirements, especially in disease states. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 600-601, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
For more Absorption, Distribution and Excretion (Complete) data for L-Valine (8 total), please visit the HSDB record page.
Hepatic
The branched-chain amino acids (BCAA) -- leucine, isoleucine, and valine -- differ from most other indispensable amino acids in that the enzymes initially responsible for their catabolism are found primarily in extrahepatic tissues. Each undergoes reversible transamination, catalyzed by a branched-chain aminotransferase (BCAT), and yields alpha-ketoisocaproate (KIC, from leucine), alpha-keto-beta-methylvalerate (KMV, from isoleucine), and alpha-ketoisovalerate (KIV, from valine). Each of these ketoacids then undergoes an irreversible, oxidative decarboxylation, catalyzed by a branchedchain ketoacid dehydrogenase (BCKAD). The latter is a multienzyme system located in mitochondrial membranes. The products of these oxidation reactions undergo further transformations to yield acetyl CoA, propionyl CoA, acetoacetate, and succinyl CoA; the BCAA are thus keto- and glucogenic.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 704, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Once the amino acid deamination products enter the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle) or the glycolytic pathway, their carbon skeletons are also available for use in biosynthetic pathways, particularly for glucose and fat. Whether glucose or fat is formed from the carbon skeleton of an amino acid depends on its point of entry into these two pathways. If they enter as acetyl-CoA, then only fat or ketone bodies can be formed. The carbon skeletons of other amino acids can, however, enter the pathways in such a way that their carbons can be used for gluconeogenesis. This is the basis for the classical nutritional description of amino acids as either ketogenic or glucogenic (ie, able to give rise to either ketones [or fat] or glucose). Some amino acids produce both products upon degradation and so are considered both ketogenic and glucogenic. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 606, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
... An NMR analysis was performed to identify the (13)C-labeled metabolites that are generated by astroglia-rich primary cultures (APC) during catabolism of U-(13)C-valine and that are subsequently released into the incubation medium. The results presented show that APC (1) are potently disposing of the valine contained in the incubation medium; (2) are capable of degrading valine to the tricarboxylic acid (TCA) cycle member succinyl-CoA; and (3) release into the extracellular milieu valine catabolites and compounds generated from them such as U-(13)C-2-oxoisovalerate, U-(13)C-3-hydroxyisobutyrate, U-(13)C-2-methylmalonate, [U-(13)C]isobutyrate, and [U-(13)C]propionate as well as several TCA cycle-dependent metabolites including lactate.
PMID:19127430 Murin R, et al; Neurochem Res 34 (7): 1195-1203 (2009).
... Metabolism of branched-chain amino acids (BCAAs) was investigated in cultured cerebellar astrocytes in a superfusion paradigm employing (15)N-labeled leucine, isoleucine, or valine. Some cultures were exposed to pulses of glutamate (50 uM; 10 sec every 2 min; 75 min in total) to mimic conditions during glutamatergic synaptic activity ... Incorporation of (15)N into intracellular glutamate from (15)N-leucine, (15)N-isoleucine, or (15)N-valine amounted to about 40-50% and differed only slightly among the individual BCAAs. Interestingly, label (%) in glutamate from (15)N-valine was not decreased upon exposure to exogenous glutamate, which was in contrast to a marked decrease in labeling (%) from (15)N-leucine or (15)N-isoleucine. This suggests an up-regulation of transamination involving only valine during repetitive exposure to glutamate. It is suggested that valine in particular might have an important function as an amino acid translocated between neuronal and astrocytic compartments, a function that might be up-regulated during synaptic activity.
PMID:17497675 Bak LK, et al; J Neurosci Res 85 (15): 3465-70 (2007).
The activities of key enzymes in the valine catabolic pathway - branched-chain aminotransferase, branched-chain alpha-keto acid dehydrogenase complex, methacrylyl (MC)-coenzyme A (CoA) hydratase (crotonase), and 3-hydroxyisobutyryl-CoA (HIB-CoA) hydrolase - were measured in normal and cirrhotic human livers. Unlike rat liver, which does not contain branched-chain aminotransferase, the aminotransferase activity in the normal liver was measurable and is increased somewhat in cirrhosis of the human liver. The total activity of branched-chain alpha-keto acid dehydrogenase complex in the normal human liver was approximately 1% of that in rat liver, and 20% to 30% of the complex was in the active form in both normal and cirrhotic livers. Only the actual activity of the enzyme was significantly decreased by cirrhosis. These results suggest that human liver is less active than rat liver in the catabolism of branched-chain amino and alpha-keto acids. Activities of MC-CoA hydratase and HIB-CoA hydrolase in human liver were very high compared with that of branched-chain alpha-keto acid dehydrogenase complex, suggesting an important role for these enzymes in catabolism of a potentially toxic compound, MC-CoA, formed as an intermediate in the catabolism of valine and isobutyrate. Cirrhosis resulted in a significant decrease in HIB-CoA hydrolase activity but had no effect on the citrate synthase activity, suggesting that the decrease in HIB-CoA hydrolase activity does not reflect a general decrease in mitochondria but that it may contribute to cellular damage that culminates in liver failure.
PMID:8938168 Taniguchi K, et al; Hepatology 24 (6): 1395-8 (1996).
(Applies to Valine, Leucine and Isoleucine)
This group of essential amino acids are identified as the branched-chain amino acids, BCAAs. Because this arrangement of carbon atoms cannot be made by humans, these amino acids are an essential element in the diet. The catabolism of all three compounds initiates in muscle and yields NADH and FADH2 which can be utilized for ATP generation. The catabolism of all three of these amino acids uses the same enzymes in the first two steps. The first step in each case is a transamination using a single BCAA aminotransferase, with a-ketoglutarate as amine acceptor. As a result, three different a-keto acids are produced and are oxidized using a common branched-chain a-keto acid dehydrogenase, yielding the three different CoA derivatives. Subsequently the metabolic pathways diverge, producing many intermediates.
The principal product from valine is propionylCoA, the glucogenic precursor of succinyl-CoA. Isoleucine catabolism terminates with production of acetylCoA and propionylCoA; thus isoleucine is both glucogenic and ketogenic. Leucine gives rise to acetylCoA and acetoacetylCoA, and is thus classified as strictly ketogenic.
There are a number of genetic diseases associated with faulty catabolism of the BCAAs. The most common defect is in the branched-chain a-keto acid dehydrogenase. Since there is only one dehydrogenase enzyme for all three amino acids, all three a-keto acids accumulate and are excreted in the urine. The disease is known as Maple syrup urine disease because of the characteristic odor of the urine in afflicted individuals. Mental retardation in these cases is extensive. Unfortunately, since these are essential amino acids, they cannot be heavily restricted in the diet; ultimately, the life of afflicted individuals is short and development is abnormal The main neurological problems are due to poor formation of myelin in the CNS.
Amino acids are selected for protein synthesis by binding with transfer RNA (tRNA) in the cell cytoplasm. The information on the amino acid sequence of each individual protein is contained in the sequence of nucleotides in the messenger RNA (mRNA) molecules, which are synthesized in the nucleus from regions of DNA by the process of transcription. The mRNA molecules then interact with various tRNA molecules attached to specific amino acids in the cytoplasm to synthesize the specific protein by linking together individual amino acids; this process, known as translation, is regulated by amino acids (e.g., leucine), and hormones. Which specific proteins are expressed in any particular cell and the relative rates at which the different cellular proteins are synthesized, are determined by the relative abundances of the different mRNAs and the availability of specific tRNA-amino acid combinations, and hence by the rate of transcription and the stability of the messages. From a nutritional and metabolic point of view, it is important to recognize that protein synthesis is a continuing process that takes place in most cells of the body. In a steady state, when neither net growth nor protein loss is occurring, protein synthesis is balanced by an equal amount of protein degradation. The major consequence of inadequate protein intakes, or diets low or lacking in specific indispensable amino acids relative to other amino acids (often termed limiting amino acids), is a shift in this balance so that rates of synthesis of some body proteins decrease while protein degradation continues, thus providing an endogenous source of those amino acids most in need. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 601-602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The mechanism of intracellular protein degradation, by which protein is hydrolyzed to free amino acids, is more complex and is not as well characterized at the mechanistic level as that of synthesis. A wide variety of different enzymes that are capable of splitting peptide bonds are present in cells. However, the bulk of cellular proteolysis seems to be shared between two multienzyme systems: the lysosomal and proteasomal systems. The lysosome is a membrane-enclosed vesicle inside the cell that contains a variety of proteolytic enzymes and operates mostly at acid pH. Volumes of the cytoplasm are engulfed (autophagy) and are then subjected to the action of the protease enzymes at high concentration. This system is thought to be relatively unselective in most cases, although it can also degrade specific intracellular proteins. The system is highly regulated by hormones such as insulin and glucocorticoids, and by amino acids. The second system is the ATP-dependent ubiquitin-proteasome system, which is present in the cytoplasm. The first step is to join molecules of ubiquitin, a basic 76-amino acid peptide, to lysine residues in the target protein. Several enzymes are involved in this process, which selectively targets proteins for degradation by a second component, the proteasome. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
72
PharmaCompass offers a list of L-VALINE API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right L-VALINE manufacturer or L-VALINE supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred L-VALINE manufacturer or L-VALINE supplier.
PharmaCompass also assists you with knowing the L-VALINE API Price utilized in the formulation of products. L-VALINE API Price is not always fixed or binding as the L-VALINE Price is obtained through a variety of data sources. The L-VALINE Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Valine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Valine, including repackagers and relabelers. The FDA regulates Valine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Valine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Valine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Valine supplier is an individual or a company that provides Valine active pharmaceutical ingredient (API) or Valine finished formulations upon request. The Valine suppliers may include Valine API manufacturers, exporters, distributors and traders.
click here to find a list of Valine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Valine DMF (Drug Master File) is a document detailing the whole manufacturing process of Valine active pharmaceutical ingredient (API) in detail. Different forms of Valine DMFs exist exist since differing nations have different regulations, such as Valine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Valine DMF submitted to regulatory agencies in the US is known as a USDMF. Valine USDMF includes data on Valine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Valine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Valine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Valine Drug Master File in Japan (Valine JDMF) empowers Valine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Valine JDMF during the approval evaluation for pharmaceutical products. At the time of Valine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Valine suppliers with JDMF on PharmaCompass.
A Valine CEP of the European Pharmacopoeia monograph is often referred to as a Valine Certificate of Suitability (COS). The purpose of a Valine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Valine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Valine to their clients by showing that a Valine CEP has been issued for it. The manufacturer submits a Valine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Valine CEP holder for the record. Additionally, the data presented in the Valine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Valine DMF.
A Valine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Valine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Valine suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Valine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Valine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Valine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Valine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Valine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Valine suppliers with NDC on PharmaCompass.
Valine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Valine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Valine GMP manufacturer or Valine GMP API supplier for your needs.
A Valine CoA (Certificate of Analysis) is a formal document that attests to Valine's compliance with Valine specifications and serves as a tool for batch-level quality control.
Valine CoA mostly includes findings from lab analyses of a specific batch. For each Valine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Valine may be tested according to a variety of international standards, such as European Pharmacopoeia (Valine EP), Valine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Valine USP).

