


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
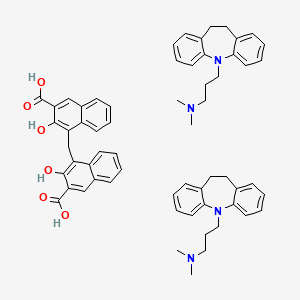

1. 4,4'-methylenebis(3-hydroxy-2-naphthoic Acid)-3-(10,11-dihydro-5h-dibenzo(b,f)azepin-5-yl)-n,n-dimethyl-1-propanamine (1:2)
2. Imidobenzyle
3. Imipramine
4. Imipramine Hydrochloride
5. Imipramine Monohydrochloride
6. Imizin
7. Janimine
8. Melipramine
9. Norchlorimipramine
10. Pryleugan
11. Tofranil
1. Imipramine Embonate
2. 10075-24-8
3. Imipramine (pamoate)
4. Mc34p30298
5. 4,4'-methylenebis(3-hydroxy-2-naphthoic) Acid, Compound With 10,11-dihydro-n,n-dimethyl-5h-dibenz(b,f)azepine-5-propylamine (1:2)
6. 4-[(3-carboxy-2-hydroxynaphthalen-1-yl)methyl]-3-hydroxynaphthalene-2-carboxylic Acid;3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-n,n-dimethylpropan-1-amine
7. Einecs 233-206-6
8. Unii-mc34p30298
9. Tofranil-pm (tn)
10. Imipramine Pamoate (usp)
11. 5h-dibenz(b,f)azepine, 5-(3-(dimethylamino)propyl)-10,11-dihydro-, Pamoate
12. Imipramine Pamoate [mi]
13. Chembl3989845
14. Schembl21834068
15. Dtxsid80143484
16. Imipramine Pamoate [vandf]
17. Imipramine Embonate [mart.]
18. Imipramine Pamoate [usp-rs]
19. Imipramine Embonate [who-dd]
20. Imipramine Pamoate [orange Book]
21. 2-naphthalenecarboxylic Acid, 4,4'-methylenebis(3-hydroxy-, Compd. With 10,11-dihydro-n,n-dimethyl-5h-dibenz(b,f)azepine-5-propanamine (1:2)
22. Imipramine Pamoate [usp Monograph]
23. Hy-107956
24. Cs-0030995
25. D08071
26. Q27283837
27. 2-naphthalenecarboxylic Acid, 4,4'-methylenebis(3-hydroxy-, Compd. With 10,11-dihydro-n,n-2-naphthalenecarboxylic Acid, 4,4'-methylenebis(3-hydroxy-, Compd. With 10,11-dihydro-n,n-dimethyl-5h-dibenz(b,f)azepine-5-propanamine (1:2)
| Molecular Weight | 949.2 g/mol |
|---|---|
| Molecular Formula | C61H64N4O6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 948.48258577 g/mol |
| Monoisotopic Mass | 948.48258577 g/mol |
| Topological Polar Surface Area | 128 Ų |
| Heavy Atom Count | 71 |
| Formal Charge | 0 |
| Complexity | 859 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)


