



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
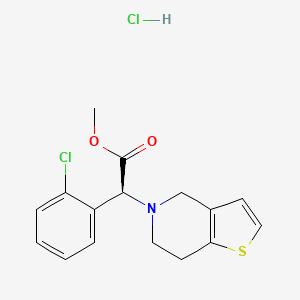

1. Clopidogrel
2. Clopidogrel Besilate
3. Clopidogrel Besylate
4. Clopidogrel Bisulfate
5. Clopidogrel Mepha
6. Clopidogrel Napadisilate
7. Clopidogrel Sandoz
8. Clopidogrel, (+)(s)-isomer
9. Clopidogrel-mepha
10. Iscover
11. Pcr 4099
12. Pcr-4099
13. Plavix
14. Sc 25989c
15. Sc 25990c
16. Sr 25989
1. 120202-65-5
2. 426o7xws6y
3. Clopidogrel (tn)
4. Unii-426o7xws6y
5. Clopidogrel Hcl
6. Clopidogrel-hcs
7. Clopidogrel-tad
8. Clopidogrel-dura
9. Clopidogrel-hydrochlorid
10. Clopidogrel-krka
11. Clopidogrel Mylan
12. Clopidogrel Qualimed
13. Clopidogrel Teva Pharma
14. Schembl1031047
15. Dtxsid40152734
16. Clopidogrel Teva Generics B.v.
17. Clopidogrel Hydrochloride [who-dd]
18. Clopidogrel Hydrochloride [ema Epar]
19. Cs-0165212
20. Clopidogrel Hydrochloride [ep Monograph]
21. D10823
22. Q27258500
23. (s)-methyl 2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl)acetate Hydrochloride? (clopidogrel Impurity Pound(c)
24. Methyl (2s)-2-(2-chlorophenyl)-2-(6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl)acetate;hydrochloride
25. Methyl-(s)-(+)-(2-chlorophenyl)-2-(6,7-dihydro-4h-thieno[3,2-c]pyridine-5-yl)-acetate Hydrochloride
26. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, .alpha.-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, Hydrochloride, (s)-
| Molecular Weight | 358.3 g/mol |
|---|---|
| Molecular Formula | C16H17Cl2NO2S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 357.0357053 g/mol |
| Monoisotopic Mass | 357.0357053 g/mol |
| Topological Polar Surface Area | 57.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 381 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Secondary prevention of atherothrombotic events
Clopidogrel is indicated in:
- Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Adult patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
In patients with moderate to high-risk Transient Ischaemic Attack (TIA) or minor Ischaemic Stroke (IS)
Clopidogrel in combination with ASA is indicated in:
- Adult patients with moderate to high-risk TIA (ABCD2 score 4) or minor IS (NIHSS 3) within 24 hours of either the TIA or IS event.
Prevention of atherothrombotic and thromboembolic events in atrial fibrillation
In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with Vitamin K antagonists (VKA) and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
For further information please refer to section 5. 1.
Clopidogrel is indicated in adults for the prevention of atherothrombotic events in:
- Patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
For further information please refer to section 5. 1.
Clopidogrel is indicated in adults for the prevention of atherothrombotic events in:
- Patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
For further information please refer to section 5. 1.
Purinergic P2Y Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P2Y RECEPTORS. Included under this heading are antagonists for specific P2Y receptor subtypes. (See all compounds classified as Purinergic P2Y Receptor Antagonists.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B01AC04
B01AC06
B01AC04


