
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
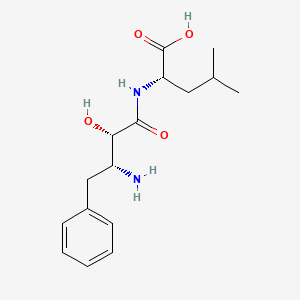

1. (3-amino-2-hydroxy-4-phenylbutanoyl)-l-leucine
2. (s-(r*,s*))-n-(3-amino-2-hydroxy-4-phenylbutyroyl)-l-leucine
3. 3-amino-2-hydroxy-4-phenylbutyryl-l-leucine
4. Bestatin
5. Bestatin, (d-leu)-(r-(r*,r*))-isomer
6. Bestatin, (d-leu)-(r-(r*,s*))-isomer
7. Bestatin, (d-leu)-(s-(r*,s*))-isomer
8. Bestatin, (l-leu)-(r-(r*,r*))-isomer
9. Bestatin, (l-leu)-(r-(r*,s*))-isomer
10. Bestatin, (l-leu)-(s-(r*,r*))-isomer
11. Bestatin, (l-leu)-(s-(r*,s*))-isomer, Hydrochloride
1. Bestatin
2. 58970-76-6
3. (s)-2-((2s,3r)-3-amino-2-hydroxy-4-phenylbutanamido)-4-methylpentanoic Acid
4. Ubenimex (bestatin)
5. Nk-421
6. (-)-bestatin
7. N-[(2s,3r)-3-amino-2-hydroxy-4-phenylbutyryl]-l-leucine
8. Chembl29292
9. N-[(2s,3r)-3-amino-2-hydroxy-1-oxo-4-phenylbutyl]-l-leucine
10. 58970-76-6 (free Base)
11. I0j33n5627
12. Nsc-265489
13. ((2s,3r)-3-amino-2-hydroxy-4-phenylbutanoyl)-l-leucine
14. L-leucine, N-[(2s,3r)-3-amino-2-hydroxy-1-oxo-4-phenylbutyl]-
15. Mfcd00083262
16. (2s)-2-[(2s,3r)-3-amino-2-hydroxy-4-phenylbutanamido]-4-methylpentanoic Acid
17. 2-(3-amino-2-hydroxy-4-phenyl-butyrylamino)-4-methyl-pentanoic Acid
18. Ubenimexum [latin]
19. Ubenimexum
20. Ubenimex [inn:jan]
21. Smr000059165
22. (2s)-2-[[(2s,3r)-3-amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic Acid
23. Einecs 261-529-2
24. Nk 421
25. Nsc 265489
26. Brn 4704312
27. Ubestatin
28. Ubenimex, Solid
29. L-leucine, N-((2s,3r)-3-amino-2-hydroxy-1-oxo-4-phenylbutyl)-
30. Unii-i0j33n5627
31. 1txr
32. Ubenimex- Bio-x
33. Ubenimex(bestatin)
34. Ubenimex, Bestatin
35. Ubenimex,(s)
36. 3-(r)-amino-2-(s)-hydroxy-4-phenylbutanoyl-(s)-leucine
37. Ubenimex [inn]
38. Ubenimex [jan]
39. Ubenimex [mi]
40. Ubenimex [mart.]
41. Ubenimex [who-dd]
42. Schembl7944
43. (3-amino-2-hydroxy-4-phenylbutanoyl)-l-leucine
44. Lopac0_000214
45. Bspbio_001553
46. Kbiogr_000273
47. Kbioss_000273
48. Mls000028649
49. Mls001424177
50. Mls006010137
51. Bcbcmap01_000178
52. Bdbm23971
53. Kbio2_000273
54. Kbio2_002841
55. Kbio2_005409
56. Kbio3_000545
57. Kbio3_000546
58. Bio2_000273
59. Bio2_000753
60. Hms1361n15
61. Hms1791n15
62. Hms1989n15
63. Hms2052a03
64. Hms2232h13
65. Hms3268o06
66. Hms3402n15
67. Hms3412n16
68. Hms3676n16
69. Hms3715b20
70. (-)-n-((2s,3r)-3-amino-2-hydroxy-4-phenylbutyryl)-l-leucine
71. (s-(r*,s*))-n-(3-amino-2-hydroxy-4-phenylbutyroyl)-l-leucine
72. Ex-a1292
73. Hy-b0134
74. Zinc1542895
75. Bdbm50367209
76. Nsc779839
77. S1591
78. Akos016339610
79. L-leucine, N-(3-amino-2-hydroxy-1-oxo-4-phenylbutyl)-, (s-(r*,s*))-
80. Ccg-101071
81. Cs-1910
82. Db03424
83. Ks-5207
84. Nc00321
85. Nsc-779839
86. Sdccgsbi-0050202.p003
87. Idi1_034023
88. Smp1_000030
89. Ncgc00025323-01
90. Ncgc00025323-02
91. Ncgc00025323-03
92. Ncgc00025323-04
93. Ncgc00025323-05
94. Ncgc00025323-06
95. Ncgc00025323-08
96. Ncgc00025323-14
97. Bu164499
98. Bcp0726000308
99. U0111
100. Ab00698345_05
101. 970b766
102. A832093
103. Sr-01000075724-3
104. W-105351
105. [(2s,3r)-3-amino-2-hydroxy-4-phenylbutanoyl]-leu
106. Brd-k59574735-001-02-7
107. Brd-k59574735-001-05-0
108. Q10909912
109. N-[(2s,3r)-3-amino-2-hydroxy-4-phenylbutanoyl]-l-leucine
110. N-((2s,3r)-3-amino-2-hydroxy-4-phenylbutanoyl)-leucine
111. N-[(2s,3r)-3-amino-2-hydroxy-4-phenylbutyryl]-l-leucine, 97%
112. (2s)-2-[[(2s,3r)-3-amino-2-hydroxy-4-phenyl-butanoyl]amino]-4-methyl-pentanoic Acid
113. (s)-2-((2s,3r)-3-amino-2-hydroxy-4-phenylbutanamido)-4-methylpentanoicacid
| Molecular Weight | 308.37 g/mol |
|---|---|
| Molecular Formula | C16H24N2O4 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 308.17360725 g/mol |
| Monoisotopic Mass | 308.17360725 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 367 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
An adjuvant therapy used for acute and chronic myelonous leukemia, lung cancer and nasopharyngeal cancer. It is also used to treat hypercholesterolaemia.
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Immunologic Factors
Biologically active substances whose activities affect or play a role in the functioning of the immune system. (See all compounds classified as Immunologic Factors.)
ABOUT THIS PAGE
50
PharmaCompass offers a list of ubenimex API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right ubenimex manufacturer or ubenimex supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred ubenimex manufacturer or ubenimex supplier.
PharmaCompass also assists you with knowing the ubenimex API Price utilized in the formulation of products. ubenimex API Price is not always fixed or binding as the ubenimex Price is obtained through a variety of data sources. The ubenimex Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ubenimex manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ubenimex, including repackagers and relabelers. The FDA regulates Ubenimex manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ubenimex API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ubenimex manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ubenimex supplier is an individual or a company that provides Ubenimex active pharmaceutical ingredient (API) or Ubenimex finished formulations upon request. The Ubenimex suppliers may include Ubenimex API manufacturers, exporters, distributors and traders.
click here to find a list of Ubenimex suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ubenimex DMF (Drug Master File) is a document detailing the whole manufacturing process of Ubenimex active pharmaceutical ingredient (API) in detail. Different forms of Ubenimex DMFs exist exist since differing nations have different regulations, such as Ubenimex USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ubenimex DMF submitted to regulatory agencies in the US is known as a USDMF. Ubenimex USDMF includes data on Ubenimex's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ubenimex USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ubenimex suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Ubenimex Drug Master File in Japan (Ubenimex JDMF) empowers Ubenimex API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Ubenimex JDMF during the approval evaluation for pharmaceutical products. At the time of Ubenimex JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Ubenimex suppliers with JDMF on PharmaCompass.
Ubenimex Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ubenimex GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ubenimex GMP manufacturer or Ubenimex GMP API supplier for your needs.
A Ubenimex CoA (Certificate of Analysis) is a formal document that attests to Ubenimex's compliance with Ubenimex specifications and serves as a tool for batch-level quality control.
Ubenimex CoA mostly includes findings from lab analyses of a specific batch. For each Ubenimex CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ubenimex may be tested according to a variety of international standards, such as European Pharmacopoeia (Ubenimex EP), Ubenimex JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ubenimex USP).

