
Synopsis
0
VMF
0
Canada
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
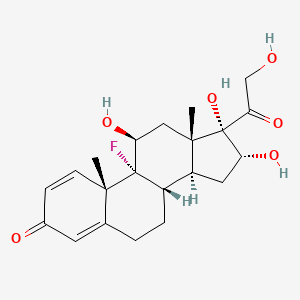

1. Aristocort
2. Volon
1. 124-94-7
2. Fluoxyprednisolone
3. Aristocort
4. Kenacort
5. Rodinolone
6. Triamcinolon
7. Volon
8. Adcortyl
9. Delphicort
10. Ledercort
11. Triamcet
12. Tricortale
13. Celeste
14. Triam-tablinen
15. Cinolone-t
16. Sk-triamcinolone
17. Triamcinlon
18. Fluoxiprednisolone
19. Triamcinolonum [inn]
20. Triamcinalone
21. Triamcinolona
22. Triamcinolonum
23. Tiamcinolonum [inn-latin]
24. Triamcinolona [inn-spanish]
25. Kenacort-ag
26. 9alpha-fluoro-16alpha-hydroxyprednisolone
27. Cl 19823
28. Mycolog
29. (8s,9r,10s,11s,13s,14s,16r,17s)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
30. 9-fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
31. Nsc-13397
32. Omcilon
33. 1zk20vi6ty
34. Aristocort Tablets
35. Mls000028542
36. Mls001066543
37. Chebi:9667
38. (8s,9r,10s,11s,13s,14s,16r,17s)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
39. 124-94-7 (free)
40. 9.alpha.-fluoro-16.alpha.-hydroxyprednisolone
41. Nsc13397
42. 9alpha-fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
43. Pregna-1,4-diene-3,20-dione,9-fluoro-11,16,17,21-tetrahydroxy-, (11b,16a)-
44. Tiamcinolonum
45. Smr000058333
46. 9-fluoro-11,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
47. Prednisolone, 9-fluoro-16.alpha.-hydroxy-
48. Nsc 13397
49. Dsstox_cid_20742
50. Dsstox_rid_79584
51. Dsstox_gsid_40742
52. 9alpha-fluoro-11beta,16alpha,17,21-tetrahydroxy-1,4-pregnadiene-3,20-dione
53. 11beta,16alpha,17alpha,21-tetrahydroxy-9alpha-fluoro-1,4-pregnadiene-3,20-dione
54. 9alpha-fluoro-11beta,16alpha,17alpha,21-tetrahydroxypregna-1,4-diene-3,20-dione
55. Kenacort (tn)
56. Triamcinolone (aristocort)
57. Hsdb 3194
58. 9-alpha-fluoro-16-alpha-hydroxyprednisolone
59. Einecs 204-718-7
60. Unii-1zk20vi6ty
61. Brn 2341955
62. Prednisolone, 9-fluoro-16alpha-hydroxy-
63. 9.alpha.-fluoro-11.beta.,16.alpha.,17,21-tetrahydroxy-1,4-pregnadiene-3,20-dione
64. 9.alpha.-fluoro-11.beta.,16.alpha.,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
65. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11.beta.,16.alpha.)-
66. Ncgc00094799-01
67. Cas-124-94-7
68. Triamcinolone [usp:inn:ban:jan]
69. Prestwick_438
70. 9-alpha-fluoro-11-beta,16-alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
71. 11-beta,16-alpha,17-alpha,21-tetrahydroxy-9-alpha-fluoro-1,4-pregnadiene-3,20-dione
72. Triamcinolone, Topical
73. Prestwick0_000120
74. Prestwick1_000120
75. Prestwick2_000120
76. Prestwick3_000120
77. Triamcinolone [mi]
78. Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,16alpha,17,21-tetrahydroxy-
79. Triamcinolone [inn]
80. Triamcinolone [jan]
81. Schembl4447
82. Chembl1451
83. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11-beta,16-alpha)-
84. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11beta,16alpha)
85. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11beta,16alpha)-
86. Triamcinolone [hsdb]
87. Lopac0_001179
88. Bspbio_000140
89. Triamcinolone [vandf]
90. 4-08-00-03629 (beilstein Handbook Reference)
91. Cid_31307
92. Mls002695935
93. Triamcinolone [mart.]
94. Spbio_002079
95. Triamcinolone [usp-rs]
96. Triamcinolone [who-dd]
97. Bpbio1_000154
98. Gtpl2870
99. Dtxsid1040742
100. Bdbm41132
101. Triamcinolone (jp17/usp/inn)
102. Hms1568g22
103. Hms2090d12
104. Hms2095g22
105. Hms2231e20
106. Hms3263l19
107. Hms3712g22
108. Triamcinolone [green Book]
109. Triamcinolone [orange Book]
110. Bcp11941
111. Ex-a4109
112. Hy-b0328
113. Zinc3882036
114. Tox21_111332
115. Tox21_300178
116. Tox21_501179
117. Triamcinolone [ep Monograph]
118. S1933
119. Triamcinolone [usp Monograph]
120. Akos015895436
121. Tox21_111332_1
122. Ac-2072
123. Ccg-205253
124. Db00620
125. Lp01179
126. Sdccgsbi-0051146.p002
127. (11beta,16alpha)-9-fluoro-11,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
128. 11.beta.,16.alpha.,17.alpha., 21-tetrahydroxy-9.alpha.-fluoro-1,4-pregnadiene-3,20-dione
129. Brn-2341955
130. Smp1_000300
131. Ncgc00021580-03
132. Ncgc00021580-04
133. Ncgc00021580-05
134. Ncgc00021580-06
135. Ncgc00021580-07
136. Ncgc00021580-08
137. Ncgc00021580-16
138. Ncgc00178404-03
139. Ncgc00254049-01
140. Ncgc00261864-01
141. 51855-44-8
142. As-13657
143. Nci60_000750
144. Eu-0101179
145. D00385
146. E70344
147. 124t947
148. Sr-01000000079
149. Q1074056
150. Sr-01000000079-3
151. Brd-k77554836-001-03-3
152. Brd-k77554836-001-14-0
153. Triamcinolone Acetonide Impurity A [ep Impurity]
154. Triamcinolone, British Pharmacopoeia (bp) Reference Standard
155. Triamcinolone, European Pharmacopoeia (ep) Reference Standard
156. Wln: L E5 B666 Ov Ku Mutj A1 Bf Cq E1 Fv1q Fq Gq
157. Triamcinolone, United States Pharmacopeia (usp) Reference Standard
158. 3-[2[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-piperidine
159. 9.alpha.-fluoro-11.beta.,17,21-tetrahydroxy-1,4-pregnadiene-3,20-dione
160. 9.alpha.-fluoro-11.beta.,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
161. Pregna-1,20-dione, 9-fluoro-11.beta.,16.alpha.,17,21-tetrahydroxy-
162. 11.beta.,17.alpha.,21-tetrahydroxy-9.alpha.-fluoro-1,4-pregnadiene-3,20-dione
163. 9-fluoro-11,beta,,16.alpha.,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
164. 9.alpha.-fluoro-11.beta.,17.alpha.,21-tetrahydroxypregna-1,4-diene-3,20-dione
165. Pregna-1,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11.beta.,16.alpha.)-
166. Pregna-1,4-diene-3, 20-dione, 9-fluoro-11.beta.,16.alpha.,17,21-tetrahydroxy-
167. (1r,2s,10s,11s,13r,14s,15s,17s)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
168. 9.alpha.-fluoro-11.beta.,16.alpha.,17.alpha., 21-tetrahydroxypregna-1,4-diene-3,20-dione
169. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11.beta.,16.alpha.)
170. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,16,17,21-tetrahydroxy-, (11beta,16alpha)-, Tetrahydro Deriv.
| Molecular Weight | 394.4 g/mol |
|---|---|
| Molecular Formula | C21H27FO6 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 394.17916674 g/mol |
| Monoisotopic Mass | 394.17916674 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 807 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Steroidal; Glucocorticoids, Synthetic; Glucocorticoids, Topical
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): ... Triamcinolone acetonide /is/ effective /in the treatment of acute traumatic synovitis and capsulitis in horses/ with no deleterious side effects.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 861
... Triamcinolone /is/ indicated as primary maintenance treatment in patients with persistent symptoms of chronic bronchial asthma. Treatment with inhaled corticosteroids is indicated in asthmatic patients whose conditions require anti-inflammatory treatment and in patients dependent on oral corticosteroids who may benefit from a gradual withdrawal from oral corticosteroids to decrease the likelihood of side effects. Regular, continuous use of inhaled corticosteroids controls chronic airway inflammation, decreases airway hyperresponsiveness, prevents asthma symptoms, reduces the frequency of asthma exacerbations, and reduces hospital admissions for asthma. Clinical studies have also reported that regular use with inhaled corticosteroids is associated with decreased mortality. Inhaled corticosteroids are effective in all types of asthma and in patients of all ages. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 889
Triamcinolone shares the actions of the other topical corticosteroids and is used for the relief of the inflammatory manifestations of corticosteroid-responsive dermatoses. The drug is also used as a paste for adjunctive treatment to provide temporary relief of symptoms associated with oral inflammatory or ulcerative lesions resulting from trauma.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3535
For more Therapeutic Uses (Complete) data for TRIAMCINOLONE (27 total), please visit the HSDB record page.
Triamcinolone acetonide oral inhalation therapy should not be used in the treatment of nonasthmatic bronchitis. Orally inhaled triamcinolone acetonide should not be used for the primary treatment of severe acute asthmatic attacks or status asthmaticus when intensive measures (e.g., oxygen, parenteral bronchodilators, IV corticosteroids) are required. Triamcinolone acetonide oral inhaler is not a bronchodilator, and patients should be warned that the drug should not be used for rapid relief of bronchospasm. /Triamcinolone acetonide/
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3058
Patients who are taking immunosuppressant drugs have increased susceptibility to infections compared with healthy individuals, and certain infections (e.g., varicella [chickenpox], measles) can have a more serious or even fatal outcome in such patients, particularly in children. In patients who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. If exposure to varicella or measles occurs in such individuals, administration of varicella zoster immune globulin (VZIG) or immune globulin, respectively, may be indicated. If varicella develops, treatment with an antiviral agent may be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2835
Patients who have received systemic corticosteroids for prolonged periods and are being switched to treatment with intranasal triamcinolone acetonide should be carefully monitored, since corticosteroid withdrawal symptoms (e.g., joint pain, muscular pain, lassitude, depression), acute adrenal insufficiency, or severe symptomatic exacerbation of asthma or other clinical conditions may occur. Systemic corticosteroid dosage should be tapered, and patients should be carefully monitored during dosage reduction. In general, the greater the dosage and duration of systemic corticosteroid therapy, the greater the time required for withdrawal of systemic corticosteroids and replacement by intranasal corticosteroids.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2835
Adverse effects occurring in at least 2% of patients receiving triamcinolone acetonide nasal aqueous suspension (Nasacort AQ) in clinical trials and more frequently than with placebo included pharyngitis, epistaxis, and increased cough.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2835
For more Drug Warnings (Complete) data for TRIAMCINOLONE (46 total), please visit the HSDB record page.
Triamcinolone hexacetonide injections are indicated for intralesional administration in alopecia areata, discoid lupus erythematosus, keloids, and necrobiosis lipoidica diabeticorum. This formulation can also be used for localized hypertrophic infiltrated inflammatory lesions of granuloma annulare, lichen planus, lichen simplex chronicus, and psoriatic plaques. Triamcinolone acetonide spray and cream are indicated for the treatment of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. A triamcinolone acetonide 10mg/mL or 40mg/mL injection is indicated intra-articularly for acute gouty arthritis, acute and subacute bursitis, acute nonspecific tenosynovitis, epicondylitis, rheumatoid arthritis, and synovitis of osteoarthritis. The same 10mg/mL injection is indicated by the intralesional route for the treatment of alopecia areata, discoid lupus erythematosus, keloids, necrobiosis lipoidica diabeticorum, and tumors of an aponeurosis or tendon. This formulation can also be used for localized hypertrophic infiltrated inflammatory lesions of granuloma annulare, lichen planus, lichen simplex chronicus, and psoriatic plaques. The 40mg/mL injection is indicated intramuscularly for controlling severe allergic conditions such as asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity, perennial or seasonal allergic rhinitis, serum sickness, and transfusion reactions; treatment of bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, Stevens-Johnson syndrome, congenital adrenal hyperplasia, hypercalcemia in cancer, nonsuppurative thyroiditis, autoimmune hemolytic anemia, Diamond-Blackfan anemia, pure red cell aplasia, secondary thrombocytopenia, trichinosis, tuberculous meningitis, acute exacerbations of multiple sclerosis or cerebral edema, sympathetic ophthalmia, temporal arteritis, uveitis, ocular inflammation, berylliosis, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis, dermatomyositis, polymyositis, and systemic lupus erythematosus; adjunct treatment of adrenocortical insufficiency, regional enteritis, ulcerative colitis, fulminating or disseminated pulmonary tuberculosis, acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis; palliative management of leukemia and lymphoma; induction of diuresis or remission of proteinuria in idiopathic nephrotic syndrome or lupus erythematosus. A triamcinolone intravitreal injection is indicated for the treatment of sympathetic ophthalmia, temporal arteritis, uveitis, and ocular inflammatory conditions. The intravitreal injection is also used for visualization during vitrectomy. An extended release suspension is indicated intra-articularly for management of pain in osteoarthritis of the knee. A triamcinolone acetonide suspension for injection into the suprachoroidal space is indicated for the treatment of macular edema associated with uveitis.
FDA Label
Triamcinolone is a corticosteroid with anti-inflammatory properties. These properties are used to treat inflammation in conditions that affect various organs and tissues. Triamcinolone should not be administered as an epidural injection.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
H02AB08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AC - Corticosteroids for local oral treatment
A01AC01 - Triamcinolone
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA12 - Triamcinolone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AB - Corticosteroids, moderately potent (group ii)
D07AB09 - Triamcinolone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XB - Corticosteroids, moderately potent, other combinations
D07XB02 - Triamcinolone
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02A - Corticosteroids for systemic use, plain
H02AB - Glucocorticoids
H02AB08 - Triamcinolone
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AD - Corticosteroids
R01AD11 - Triamcinolone
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BA - Glucocorticoids
R03BA06 - Triamcinolone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA05 - Triamcinolone
Absorption
A 16mg oral dose of triamcinolone reaches a Cmax of 5.230.84ng/mL with a Tmax of 2.240.78h and an AUC of 36.06.2ng\*h/mL. A 2mg intravenous dose of triamcinolone acetonide has an AUC of 57.7ng\*h/mL. The bioavailability of 800g of inhaled triamcinolone acetonide is 25%, with 10.4% coming from pulmonary absorption and the rest being accounted for by deposition on the oral mucosa and other underlying factors. An inhaled dose of triamcinolone acetonide reaches a Cmax of 0.92ng/mL with a Tmax of 1.74h and an AUC of 5.12ng\*h/mL. The fraction of an inhaled dose that is actually absorbed via the pulmonary route reaches a Cmax of 0.55ng/mL with a Tmax of 0.66h and an AUC of 2.15ng\*h/mL. A 16mg oral dose of triamcinolone diacetate reaches a Cmax of 5.331.55ng/mL with a Tmax of 1.860.47h and an AUC of 32.79.9ng\*h/mL.
Route of Elimination
Approximately 20% of a dose of triamcinolone is recovered in the urine as the unchanged drug, 25% is recovered as 6-beta-hydroxy-triamcinolone, and 5% is recovered as unidentified metabolites.
Volume of Distribution
The apparent volume of distribution of triamcinolone is 115.210L. The mean apparent volume of distribution of triamcinolone acetonide is 1.96L/kg. The apparent volume of distribution of triamcinolone diacetate is 119.733.14L.
Clearance
The clearance of triamcinolone is 28.65.6L/h. The mean total body clearance of triamcinolone acetonide is 0.57L/h. The clearance of triamcinolone diacetate is 34.410.6L/h.
TOPICAL APPLICATIONS OF CREAM...CONTAINING...[(14)C]TRIAMCINOLONE ACETONIDE... IN RABBIT. ...9%...OF (14)C WERE ABSORBED FROM OCCLUDED/ABRADED SKIN, THROUGH WHICH PERCUTANEOUS ABSORPTION WOULD BE MAXIMALLY ENHANCED. /TRIAMCINOLONE ACETONIDE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 128
The absorption, distribution and metabolic fate of triamcinolone acetonide-(14)C-21-phosphate were studied in the dog, monkey, and rat. A comparison of levels of radioactivity in blood or plasma, reached after intramuscular or intravenous administration, indicated that the drug was completely absorbed from the site of intramuscular injection within 10-15 min in all three species. Within 1-5 min after intramuscular or intravenous administration, the 21-phosphate ester was completely hydrolyzed to triamcinolone acetonide, which was present in the blood. The radioactivity was eliminated rapidly (t1/2 = 1-2 hr) from plasma (dogs, monkeys, and rats) and tissues (rats) after intramuscular or intravenous administration. In the three species, the major route of excretion was via the bile; however, the ratio of biliary to urinary excretion among the species varied considerably (from 1.5 to 15). In rats, excretion of radioactivity as expired carbon dioxide accounted for only 2-3 percent of the dose. 6beta-Hydroxytriamcinolone acetonide was the major metabolite in urine of the three species. Hydrolytic cleavage of the acetonide group did not appear to be significant.
PMID:807712 Kripalani KJ et al; J Pharm Sci 64 (8): 1351-9 (1975)
Six healthy male subjects each received a single 100 uCi (approximately 800 ug) oral dose of (14)C-triamcinolone acetonide. Plasma, urine, and fecal samples were collected at selected times and analyzed for triamcinolone acetonide and (14)C-derived radioactivity. Plasma protein binding of triamcinolone acetonide was also determined. Metabolite profiling and identification were carried out in plasma and excreta. Principle metabolites were assessed for activity with in vitro anti-inflammatory models. (14)C-triamcinolone acetonide was found to be systemically absorbed following oral administration. The presystemic metabolism and clearance of triamcinolone acetonide were extensive, with only a small fraction of the total plasma radioactivity being made up of triamcinolone acetonide. Little to no parent compound was detected in the plasma 24 hours after administration. Most of the urinary and fecally (14)C-derived radioactivity was also excreted within 24 and 72 hours postdose, respectively. Mean plasma protein binding of triamcinolone acetonide was constant, predictable, and a relatively low 68% over a 24-fold range of plasma concentrations. Three principle metabolites of triamcinolone acetonide were profiled in plasma, urine, and feces. These metabolites were identified as 6 beta-hydroxy triamcinolone, 21-carboxylic acid triamcinolone acetonide, and 6 beta-hydroxy-21-oic triamcinolone acetonide. All three metabolites failed to show any concentration-dependent effects in anti-inflammatory models evaluating IL-5-sustained eosinophil viability and IgE-induced basophil histamine release. /Triamcinolone acetonide/
PMID:10883419 Argenti D et al; J Clin Pharmacol 40 (7): 770-80 (2000)
Triamcinolone acetonide is a glucocorticoid administered by oral inhalation in the management of asthma. With oral inhalation of glucocorticoids, systemic absorption can come from oropharyngeal, gastrointestinal, or airway deposition of the drug. The objectives of this study were to determine the absolute bioavailability of triamcinolone acetonide following inhalation administration and to delineate the airway contribution of triamcinolone acetonide absorption relative to the absolute bioavailability. All subjects received a 5-minute 400 mcg intravenous infusion of triamcinolone acetonide and a single 800 mcg dose of inhaled triamcinolone acetonide with and without oral charcoal administration in a randomized three-way crossover fashion. The oral charcoal allowed for isolating the pulmonary component of absorption by adsorbing the oropharyngeal and gastrointestinal deposited drug. The mean (+/- SD) absolute bioavailability value for inhaled triamcinolone acetonide was 25% (8.75%). Delineation of the airway contribution of triamcinolone acetonide absorption showed that 10.4% of an inhaled dose is absorbed as triamcinolone acetonide from the lungs. Mean (+/- SD) total body clearance was rapid at 0.57 (0.12) L/hr/kg. The mean (+/- SD) apparent volume of distribution following the intravenous dose was a low 1.96 (0.31) L/kg. No significant differences were noted in the apparent terminal elimination half-life of triamcinolone acetonide (approximately 2.4 hr) between treatments.
PMID:10392324 Argenti D et al; J Clin Pharmacol 39 (7): 695-702 (1999)
For more Absorption, Distribution and Excretion (Complete) data for TRIAMCINOLONE (9 total), please visit the HSDB record page.
The major metabolite of triamcinolone is 6-beta-hydroxy-triamcinolone. Data regarding the metabolism of triamcinolone is not readily available.
Hepatic to 3 less active metabolites, 6-beta-hydroxytriamcinolone acetonide, 21-carboxytriamcinolone acetonide, and 21-carboxy-6-beta-hydroxytriamcinolone acetonide. /Triamcinolone acetonide/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 898
The half life of triamcinolone is 2.7h. The mean terminal elimination half life following an inhaled dose of triamcinolone acetonide is 2.4h. The half life of triamcinolone diacetate is 2.8h.
Intravenous: Approximately 90 minutes (plasma). Intranasal: Apparent half-life is 4 hours (plasma) (range, 1 to 7 hours); however, this value probably reflects lingering absorption; 3.1 hours with aqueous formulation. /Triamcinolone acetonide/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 898
88 minutes (plasma) NOTE: The plasma half-life of the inhaled corticosteroids does not correspond well with the biologic half-life.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 890
Corticosteroids like triamcinolone inhibit phospholipase A2 on cell membranes, preventing the breakdown of lysosomal membranes of leukocytes, which in turn prevent the formation of arachidonic acid, which decrease expression of cyclooxygenase and lipoxygenase, inhibiting synthesis of prostaglandins and leukotrienes. Anti-inflammatory activity occurs via reversal of vascular dilation and reducing permeability, which prevents macrophage and leukocyte migration. Triamcinolone also inhibits nuclear factor kappa-B, which decreases the production of pro-inflammatory signals such as interleukin-6, interleukin-8, and monocyte chemoattractant protein-1.
Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2128
Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA (chromatin), and stimulate transcription of messenger RNA (mRNA) and subsequent protein synthesis of various inhibitory enzymes responsible for the anti-inflammatory effects of topical corticosteroids. These anti-inflammatory effects include inhibition of early processes such as edema, fibrin deposition, capillary dilatation, movement of phagocttes into the area, and phagocytic activities. Later processes, such as capillary production, collagen deposition, and keloid formation also are inhibited by corticosteroids. The overall actions of topical corticosteroids are catabolic. /Corticosteroids (topical)/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 919
The potent anti-inflammatory action may be due to an inhibition of the secretion of growth factors, endothelial activating and other cytokines from lymphocytes, eosinophils, macrophages, fibroblasts, and mast cells. The results are decreased influx of inflammatory cells into the bronchial walls, due in part to inhibition of expression of adhesion molecules on the endothelium and in the tissue. Decreased activation and survival of eosinophils in the lung tissue and a reduction in numbers of mast cells are further effects. Corticosteroids may inhibit release of mediators from basophils and enzymes from macrophages. There is decreased permeability through vasoconstriction and direct inhibition of endothelial cell contradiction. Beta-adrenergic-receptor numbers may be increased, which results in an enhanced response to beta-adrenergic bronchodilators and reduced down-regulation of beta-receptors after prolonged beta-agonist exposure. Inhaled corticosteroids also inhibit mucus secretion in airways, possibly by a direct action on submucosal gland cells and an indirect inhibitory effect caused by the reduction in inflammatory mediators that stimulate mucus secretion. The amount and viscosity of sputum are reduced. /Corticosteroids (inhalation-local/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 890

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
74
PharmaCompass offers a list of Triamcinolone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triamcinolone manufacturer or Triamcinolone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triamcinolone manufacturer or Triamcinolone supplier.
PharmaCompass also assists you with knowing the Triamcinolone API Price utilized in the formulation of products. Triamcinolone API Price is not always fixed or binding as the Triamcinolone Price is obtained through a variety of data sources. The Triamcinolone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Triamcinolone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Triamcinolone, including repackagers and relabelers. The FDA regulates Triamcinolone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Triamcinolone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Triamcinolone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Triamcinolone supplier is an individual or a company that provides Triamcinolone active pharmaceutical ingredient (API) or Triamcinolone finished formulations upon request. The Triamcinolone suppliers may include Triamcinolone API manufacturers, exporters, distributors and traders.
click here to find a list of Triamcinolone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Triamcinolone DMF (Drug Master File) is a document detailing the whole manufacturing process of Triamcinolone active pharmaceutical ingredient (API) in detail. Different forms of Triamcinolone DMFs exist exist since differing nations have different regulations, such as Triamcinolone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Triamcinolone DMF submitted to regulatory agencies in the US is known as a USDMF. Triamcinolone USDMF includes data on Triamcinolone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Triamcinolone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Triamcinolone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Triamcinolone Drug Master File in Japan (Triamcinolone JDMF) empowers Triamcinolone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Triamcinolone JDMF during the approval evaluation for pharmaceutical products. At the time of Triamcinolone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Triamcinolone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Triamcinolone Drug Master File in Korea (Triamcinolone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Triamcinolone. The MFDS reviews the Triamcinolone KDMF as part of the drug registration process and uses the information provided in the Triamcinolone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Triamcinolone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Triamcinolone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Triamcinolone suppliers with KDMF on PharmaCompass.
A Triamcinolone CEP of the European Pharmacopoeia monograph is often referred to as a Triamcinolone Certificate of Suitability (COS). The purpose of a Triamcinolone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Triamcinolone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Triamcinolone to their clients by showing that a Triamcinolone CEP has been issued for it. The manufacturer submits a Triamcinolone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Triamcinolone CEP holder for the record. Additionally, the data presented in the Triamcinolone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Triamcinolone DMF.
A Triamcinolone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Triamcinolone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Triamcinolone suppliers with CEP (COS) on PharmaCompass.
A Triamcinolone written confirmation (Triamcinolone WC) is an official document issued by a regulatory agency to a Triamcinolone manufacturer, verifying that the manufacturing facility of a Triamcinolone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Triamcinolone APIs or Triamcinolone finished pharmaceutical products to another nation, regulatory agencies frequently require a Triamcinolone WC (written confirmation) as part of the regulatory process.
click here to find a list of Triamcinolone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Triamcinolone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Triamcinolone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Triamcinolone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Triamcinolone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Triamcinolone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Triamcinolone suppliers with NDC on PharmaCompass.
Triamcinolone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Triamcinolone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Triamcinolone GMP manufacturer or Triamcinolone GMP API supplier for your needs.
A Triamcinolone CoA (Certificate of Analysis) is a formal document that attests to Triamcinolone's compliance with Triamcinolone specifications and serves as a tool for batch-level quality control.
Triamcinolone CoA mostly includes findings from lab analyses of a specific batch. For each Triamcinolone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Triamcinolone may be tested according to a variety of international standards, such as European Pharmacopoeia (Triamcinolone EP), Triamcinolone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Triamcinolone USP).

