
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
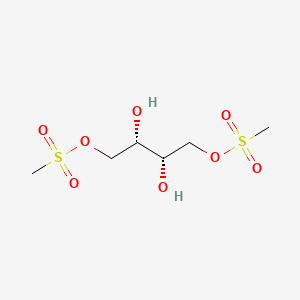

1. L-dihydroxybusulfan
2. L-threitol-1,4-bismethanesulfonate
3. Nsc 39069
4. Treosulfan, ((r*,r*)-(+-))-isomer
5. Treosulfan, (r*,r*)-isomer
6. Treosulfan, (r*,s*)-isomer
7. Treosulfan, (r-(r*,r*))-isomer
1. 299-75-2
2. Treosulphan
3. Ovastat
4. Threosulphan
5. Dihydroxybusulfan
6. Nsc 39069
7. Nsc-39069
8. (2s,3s)-threitol 1,4-bismethanesulfonate
9. Co61er3epi
10. Trecondi
11. L-threitol 1,4-dimethanesulfonate
12. Chebi:82557
13. L-threitol-1,4-bis(methanesulfonate)
14. Treosulfan (inn)
15. Treosulfan [inn]
16. Cb 40067
17. (2s,3s)-2,3-dihydroxybutane-1,4-diyl Dimethanesulfonate
18. Treosulfano
19. Treosulfanum
20. Treosulfanum [inn-latin]
21. Treosulfano [inn-spanish]
22. Ccris 2781
23. Hsdb 6963
24. Einecs 206-081-0
25. Unii-co61er3epi
26. L-threitol, 1,4-bis(methanesulfonate)
27. Threitol, 1,4-dimethanesulfonate, L-(+)-
28. Ovastat (tn)
29. L-threitol, 1,4-bismethanesulfonate
30. Grafapex
31. Graftrevo
32. Kepcondi
33. Trecondyv
34. Treograft
35. Treosulphannsc 39069
36. Treosulfan [hsdb]
37. Treosulfan [iarc]
38. Treosulfan [usan]
39. Threitol, 1,4-dimethanesulfonate, (2s,3s)-
40. Treosulfan [mart.]
41. Treosulfan [who-dd]
42. Treosulfan [usan:inn:ban]
43. Chembl455186
44. Schembl5399430
45. Dtxsid0026173
46. (s-(r*,r*))-1,2,3,4-butanetetrol, 1,4-dimethanesulfonate
47. 1,2,3,4-butanetetrol, 1,4-dimethanesulfonate, (s-(r*,r*))-
48. Who 3103
49. Zinc1671094
50. L-threitol-1,4-bis-methanesulfonate
51. Mfcd02258964
52. S6958
53. L-threitol 1,4-bis(methanesulfonate)
54. Cs-3889
55. Db11678
56. Hy-16503
57. Ps-12126
58. L-threitol 1,4-dimethanesulphonate
59. C19557
60. D07253
61. A912763
62. (2s,3s)-2,3-dihydroxy-4-(methanesulfonyloxy)butyl Methanesulfonate
63. (s)-3-boc-amino-1-diazo-3-(4-benzyloxy)phenyl-2-butanone
64. [(2s,3s)-2,3-dihydroxy-4-methylsulfonyloxybutyl] Methanesulfonate
| Molecular Weight | 278.3 g/mol |
|---|---|
| Molecular Formula | C6H14O8S2 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 278.01300975 g/mol |
| Monoisotopic Mass | 278.01300975 g/mol |
| Topological Polar Surface Area | 144 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Therapy of advanced renal cell carcinoma remains difficult. New therapeutic schemes besides cytokine treatment should be evaluated. The following study analyzes the in vitro toxicity of treosulfan on spheroids of 8 primary cultures of renal cell carcinoma cells. these data were compared to the toxicity of vinblastine. All investigations were performed in regard to the P-glycoprotein (Pgp) expression of the cells, which is one of the main causes of multidrug resistance. Four Pgp positive and four Pgp negative spheroids were incubated with the drugs in increasing doses. Toxicity was measured using the MTT toxicity assay as well as trypan blue exclusion. Significantly higher toxicity of treosulfan compared to vinblastine could be demonstrated. In addition, the effects of treosulfan were not related to Pgp expression. These results are encouraging and a phase II study analyzing the efficacy of treosulfan in patients with advanced renal cell carcinoma has been initiated in our institution.
PMID:9738287 Lugler A et al; Urologe A 37 (4): 367-71 (1998)
[Schmidt F et al; J Neurooncol 49 (3): 231-4 (2000)] Treosulfan is a bifunctional alkylating prodrug with activity against various solid tumors. To improve the outcome for patients with recurrent malignant glioma, we assessed the efficacy of intravenous treosulfan (6-10 g/m2 4-weekly) as salvage therapy for patients with recurrent or progressive glioblastoma (GB, n = 14) or anaplastic astrocytoma (AA, n = 2). All patients had prior involved-field radiotherapy and adjuvant nitrosourea-based chemotherapy. A total of 56 cycles were administered. Tumor responses were assessed radiologically and clinically prior to each cycle. All patients were assessable for toxicity, response and survival. There were no complete or partial responses (CR, PR). Two patients progressed after the first cycle, 14 patients had initially stable disease (SD). Median progression-free survival was 3.25 months for the glioblastoma patients. Five patients were progression-free at 6 months (30%), including the 2 anaplastic astrocytoma patients. The 2 anaplastic astrocytoma patients are stable at 22 months. ... Treosulfan has modest activity in patients with recurrent malignant glioma. Further evaluation of treosulfan in chemonaive malignant glioma patients is warranted.
PMID:11212902 Schmidt F et al; J Neurooncol 49 (3): 231-4 (2000)
Myelosuppression was the dose-limiting toxicity in this cohort of nitrosourea-pretreated patients.
PMID:11212902 Schmidt F et al; J Neurooncol 49 (3): 231-4 (2000)
Acute non-lymphocytic leukemia occurred in eight women following long-term treatment with Treosulfan (= dihydroxybusulfan) for ovarian carcinoma. The leukemia developed from 21 to 58 months (median 50 months) after the institution of chemotherapy. At the time when the leukemia appeared seven of the patients were in complete, and one in partial, remission as regards the ovarian carcinoma. Seven of the eight cases of acute leukemia occurred in a series of 553 patients treated with Treosulfan for ovarian cancer in the period from 1970 to 1977 and followed closely for a total of 1159 patient-years up to February 1978. As compared with an expected number of 0.04 cases of acute myeloblastic leukemia based on patient-years, the observed seven cases correspond to a 175-times increased risk. Although the cumulative probability of acute non-lymphocytic leukemia among surviving patients at five years using life-table statistics was 7.6%, the survival curve for the 553 patients with ovarian carcinoma was only slightly affected by death from leukemia. The probability of developing acute leukemia in this study was not significantly correlated to the total cumulative dosage of Treosulfan. Cytogenetic studies of the bone marrow performed after the development of acute leukemia showed chromosome abnormalities in all five patients examined, with hypodiploidy and loss of B and C group chromosomes.
PMID:7351000 Pedersen-Bjergaard J et al; Cancer 45 (1): 19-29 (1980)
Treosulfan and busulphan are similar molecules, the former used in the treatment of ovarian cancer and the latter in chronic myelogenous leukaemia. We have used both in the differential staining cytotoxicity (DiSC) assay for in vitro drug sensitivity testing to aid in the choice of chemotherapy for individual patients. It was observed that occasionally the viability of control cells in one assay box was reduced compared with control cells in other boxes from the same assay. Treosulfan was suspected as the cause because cells throughout the microtitre box containing treosulfan had reduced viability in 28/62 (45%) experiments and in 9 of these, total kill of all cells in the box was observed. We tested the hypothesis that a metabolite of treosulfan might be the cause of this airborne cytotoxicity, and found that whilst 10 mg ml-1 of either methane sulphonic acid or tetrahydrofuran had no airborne cytotoxic effect, 1 mg ml-1 diepoxybutane killed over 95% of cells in all tubes in the same box. Treosulfan is another chemical (cf. azide, mafosfamide and possibly other cytotoxic agents) that can cause airborne cytotoxicity.
PMID:7531465 Bosanquet AG, Burlton AR; cytotechnology 16 (2): 131-6 (1994)
Treosulfan in combination with fludarabine is indicated as part of conditioning treatment prior to allogeneic haematopoietic stem cell transplantation (alloHSCT) in adult patients with malignant and non malignant diseases, and in paediatric patients older than one month with malignant diseases.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L01AB02
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AB - Alkyl sulfonates
L01AB02 - Treosulfan
In a pharmacologic study of the bioavailability of treosulfan in a capsule formulation, patients with relapsed ovarian carcinoma were treated with alternating doses of oral and intravenous (i.v.) treosulfan of 1.5 or 2.0 g daily for 5 to 8 days. ... The bioavailability ratio (f) of oral to i.v. administration was calculated as 0.97 + or - 0.18 (mean + or - SD) using the values AUC oral = 82.1 + or- 39.4 ug/ml hr and AUC i.v. = 85.4 + or - 30.3 ug/ml hr. The peak plasma concentration cmax (29 + or - 14 ug/ml vs 65 + or - 23 ug/ml) was significantly (P < 0.01) higher after i.v. administration and the tmax after oral administration was 1.5 + or - 0.34 hr. The terminal half-life of treosulfan was about 1.8 hr. The mean urinary excretion of the parent compound was about 15% of the administered total dose over 24 hr (range 6-26%). ... A feasible and reliable oral treosulfan formulation could provide the basis for the development of long-term low-dose outpatient treatment of patients with malignant diseases.
Hilger RA et al; Cancer Chemother Pharmacol 45 (6): (2000)
The anti-tumour drug treosulfan (L-threitol 1,4-bismethanesulphonate, Ovastat) is a prodrug for epoxy compounds by converting non-enzymatically to L-diepoxybutane via the corresponding monoepoxide under physiological conditions. The present study supports the hypothesis that this conversion of treosulfan is required for cytotoxicity in vitro. DNA alkylation and interstrand cross-linking of plasmid DNA is observed after treosulfan treatment, but this is again produced via the epoxide species. Alkylation occurs at guanine bases with a sequence selectivity similar to other alkylating agents such as the nitrogen mustards. In treosulfan-treated K562 cells, cross-links form slowly, reaching a peak at approximately 24 h. Incubation of K562 cells with preformed epoxides shows faster and more efficient DNA cross-linking.
PMID:9888467 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2362201 Hartley JA et al; Br J Cancer 79 (2): 264-6 (1999)
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34570
Submission : 2020-06-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12914
Submission : 1998-03-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 705
Submission : 1963-10-16
Status : Inactive
Type : II


Date of Issue : 2025-09-26
Valid Till : 2028-07-02
Written Confirmation Number : WC-0226
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34570
Submission : 2020-06-29
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12914
Submission : 1998-03-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 705
Submission : 1963-10-16
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2025-09-26
Valid Till : 2028-07-02
Written Confirmation Number : WC-0226
Address of the Firm : D-24 & D-24/1, M.I.D.C., Kurkumbh, Taluka: Daund, District. Pune -413802, Mahara...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Fermion, a wholly owned subsidiary of Orion Corporation and headquartered in Espoo, Finland, is a fully integrated CDMO offering API and formulation services. Its API portfolio inc...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
95
PharmaCompass offers a list of Treosulfan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Treosulfan manufacturer or Treosulfan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Treosulfan manufacturer or Treosulfan supplier.
PharmaCompass also assists you with knowing the Treosulfan API Price utilized in the formulation of products. Treosulfan API Price is not always fixed or binding as the Treosulfan Price is obtained through a variety of data sources. The Treosulfan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Treosulphan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Treosulphan, including repackagers and relabelers. The FDA regulates Treosulphan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Treosulphan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Treosulphan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Treosulphan supplier is an individual or a company that provides Treosulphan active pharmaceutical ingredient (API) or Treosulphan finished formulations upon request. The Treosulphan suppliers may include Treosulphan API manufacturers, exporters, distributors and traders.
click here to find a list of Treosulphan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Treosulphan DMF (Drug Master File) is a document detailing the whole manufacturing process of Treosulphan active pharmaceutical ingredient (API) in detail. Different forms of Treosulphan DMFs exist exist since differing nations have different regulations, such as Treosulphan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Treosulphan DMF submitted to regulatory agencies in the US is known as a USDMF. Treosulphan USDMF includes data on Treosulphan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Treosulphan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Treosulphan suppliers with USDMF on PharmaCompass.
A Treosulphan written confirmation (Treosulphan WC) is an official document issued by a regulatory agency to a Treosulphan manufacturer, verifying that the manufacturing facility of a Treosulphan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Treosulphan APIs or Treosulphan finished pharmaceutical products to another nation, regulatory agencies frequently require a Treosulphan WC (written confirmation) as part of the regulatory process.
click here to find a list of Treosulphan suppliers with Written Confirmation (WC) on PharmaCompass.
Treosulphan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Treosulphan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Treosulphan GMP manufacturer or Treosulphan GMP API supplier for your needs.
A Treosulphan CoA (Certificate of Analysis) is a formal document that attests to Treosulphan's compliance with Treosulphan specifications and serves as a tool for batch-level quality control.
Treosulphan CoA mostly includes findings from lab analyses of a specific batch. For each Treosulphan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Treosulphan may be tested according to a variety of international standards, such as European Pharmacopoeia (Treosulphan EP), Treosulphan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Treosulphan USP).

