



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0

1. Aluminum Monostearate
2. Aluminum Tristearate
3. Ammonium Stearate
4. Calcium Stearate
5. Magnesium Stearate
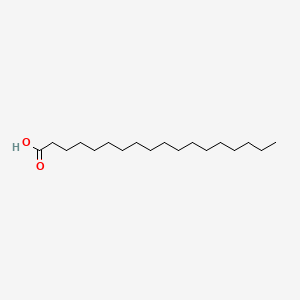
6. Octadecanoic Acid
7. Sodium Stearate
8. Zinc Stearate
1. Octadecanoic Acid
2. 57-11-4
3. N-octadecanoic Acid
4. Stearophanic Acid
5. Cetylacetic Acid
6. Pearl Stearic
7. Stearex Beads
8. 1-heptadecanecarboxylic Acid
9. Octadecansaeure
10. Stearinsaeure
11. Vanicol
12. Hydrofol Acid 150
13. Century 1240
14. Industrene R
15. Glycon Dp
16. Glycon Tp
17. Humko Industrene R
18. Dar-chem 14
19. Formula 300
20. Hydrofol 1895
21. Hystrene 7018
22. Hystrene 9718
23. Glycon S-80
24. Glycon S-90
25. Hydrofol Acid 1655
26. Hydrofol Acid 1855
27. Tegostearic 254
28. Tegostearic 255
29. Tegostearic 272
30. Hystrene 80
31. Octadecoic Acid
32. Industrene 5016
33. Hystrene S-97
34. Hystrene T-70
35. Emersol 120
36. Emersol 132
37. Hystrene 4516
38. Hystrene 5016
39. Groco 54
40. Groco 55
41. Groco 55l
42. Groco 58
43. Groco 59
44. Glycon S-70
45. Industrene 8718
46. Industrene 9018
47. Kam 1000
48. Emersol 150
49. Fema No. 3035
50. Steric Acid
51. C18:0
52. Neo-fat 18-53
53. Neo-fat 18-54
54. Neo-fat 18-59
55. Neo-fat 18
56. Acidum Stearinicul
57. Barolub Fta
58. Caswell No. 801d
59. Hy-phi 1199
60. Hy-phi 1205
61. Hy-phi 1303
62. Hy-phi 1401
63. Neo-fat 18-s
64. Kam 2000
65. Kam 3000
66. Oktadekansaeure
67. Neo-fat 18-55
68. Neo-fat 18-61
69. Acide Stearique
70. Century 1210
71. Pd 185
72. Stearic Acid 50
73. Acide Octadecanoique
74. Naa 173
75. Hydrofol Acid 150 (van)
76. Ccris 2305
77. Prifac 2918
78. Hsdb 2000
79. Vis-plus
80. Prodhygine
81. Epa Pesticide Chemical Code 079082
82. Stearic Acid Cherry
83. Edenor C18
84. Stearic Acid (tn)
85. Purified Stearic Acid
86. Dervacid 3155
87. Ch3-[ch2]16-cooh
88. Loxiol G 20
89. Adeka Sa 300
90. Century 1220
91. Century 1230
92. Emersol 6349
93. Ai3-00909
94. Lunac S 20
95. Lunac S 40
96. Wo 2 (fatty Acid)
97. Hydrofol Acid 1895
98. Octadecanoic Acid, Dimer
99. Bonderlube 235
100. Mfcd00002752
101. Nsc-25956
102. 4elv7z65ap
103. Adeka Fatty Acid Sa 910
104. Chembl46403
105. Chebi:28842
106. Nsc25956
107. Nsc-261168
108. Ncgc00091596-02
109. Dsstox_cid_1642
110. Dsstox_rid_76256
111. Dsstox_gsid_21642
112. 18639-67-3
113. Stearic Acid, Pure
114. Stearicacid
115. Lunac
116. Stearic Acid (powder/beads/flakes)
117. Cas-57-11-4
118. Isostearic Acid Ex
119. Nsc 25956
120. Haimaric Mkh(r)
121. Prisorine 3501
122. Prisorine 3502
123. Prisorine 3508
124. Emersol 153nf
125. Emersol 871
126. Emersol 875
127. Emery 875d
128. Emery 871
129. Unimac 5680
130. C-lube 10
131. Einecs 200-313-4
132. Stearic Acid [jan:nf]
133. Fatty Acids, C16-20
134. Unii-4elv7z65ap
135. Brn 0608585
136. Stearophanate
137. Promulsin
138. Stearex
139. Tsubaki
140. N-octadecanoate
141. Bassinic Acid
142. Lactaric Acid
143. Proviscol Wax
144. Talgic Acid
145. Kolliwax S Fine
146. Edenor Htict-n
147. 1hmr
148. 1hmt
149. 4fnn
150. Kiri Stearic Acid
151. Lunac Ya
152. N-octadecylic Acid
153. Palmitostearic Acid
154. Stearic Acid 70
155. Stearic Acid, Cp
156. Sterene 60b
157. Sterene 60r
158. Einecs 250-178-0
159. F 3 (lubricant)
160. Industrene 4518
161. Nonsoul Sk 1
162. Pristerene 4900
163. Pristerene 4904
164. Pristerene 4910
165. Pristerene 4916
166. Pristerene 4963
167. Pristerene 4981
168. Pristerene 9429
169. Pristerene 9559
170. Pristerine 4989
171. Hystrene S 97
172. Hystrene T 70
173. Edenor St 1
174. Sunfat 18s
175. Emersol 153
176. Selosol 920
177. Sterene 460
178. Industrene 5016k
179. Stearic Acid 110
180. Stearic Acid 120
181. Stearic Acid 420
182. Hystrene 9718nf
183. Kortacid 1895
184. Radiacid 0427
185. Edenor St 20
186. Lunac 30
187. Serfax Mt 90
188. Stearic Acid_ravikumar
189. Unister Naa 180
190. Century 1224
191. Edenor Ht-jg 60
192. Lunac S 90kc
193. Stearic Acid (8ci)
194. Stearic Acid, Puriss.
195. Hyfac 410
196. Hyfac 420
197. Hyfac 421
198. Hyfac 422
199. Hystrene 7018 Fg
200. Hystrene 9718nffg
201. Lunac S 30
202. Lunac S 50
203. Lunac S 90
204. Lunac S 98
205. Prifac 5905
206. 3v2p
207. 875d
208. 1-heptadecanecarboxylate
209. Hydrogenated Tallow Acid
210. Industrene 7018 Fg
211. Stearic Acid Nf Powder
212. Afco-chem B 65
213. Hydrogenated Tallow Acids
214. Stearic Acid - 65%
215. Stearic Acid - 70%
216. Stearic Acid 153 Nf
217. Heptadecanecarboxylic Acid
218. Stearic Acid & Glycerin
219. Edenor C 18/98
220. Tallow Acid, Hydrogenated
221. Neo-fat 18-57
222. Neo-fat 18-58
223. S 300 (fatty Acid)
224. Octadecanoic Acid (9ci)
225. Stearic Acid, >=98%
226. Schembl659
227. Hystrene 9718 Nf Fg
228. Sa 400 (fatty Acid)
229. Bmse000485
230. Stearic Acid [ii]
231. Stearic Acid [mi]
232. Stearic Acid, High Purity
233. Stearic Acid-[13c18]
234. Stearic Acid-2-[13c]
235. Ec 200-313-4
236. Emery 400 (salt/mix)
237. Stearic Acid [dsc]
238. Stearic Acid [jan]
239. Tallow, Acids, Hydrogenated
240. Stearic Acid (jp15/nf)
241. Stearic Acid (jp17/nf)
242. Stearic Acid Triple-pressed
243. Triple Pressed Stearic Acid
244. Emersol 110 (salt/mix)
245. Stearic Acid - High Purity
246. Stearic Acid [fhfi]
247. Stearic Acid [hsdb]
248. Stearic Acid [inci]
249. Stearic Acid (reagent Grade)
250. Stearic Acid [vandf]
251. 4-02-00-01206 (beilstein Handbook Reference)
252. Wln: Qv17
253. Agar Agar Type K-100 Nf
254. Stearic Acid [mart.]
255. Hydrogenated Tallow Acid, Beef
256. Hydrogenated Tallow Fatty Acids
257. Stearic Acid - Triple Pressed
258. Stearic Acid [usp-rs]
259. Stearic Acid [who-dd]
260. 17fa
261. Gtpl3377
262. Wo 2
263. Stearic Acid (fragrance Grade)
264. Stearic Acid High Purity 90%
265. Stearic Acid-1,2-[13c2]
266. Dtxsid8021642
267. Fatty Acids, Hydrogenated Tallow
268. Tallow Fatty Acids, Hydrogenated
269. Tallow, Hydrogenated Fatty Acids
270. Unii-x33r8u0062
271. Nonsoul Sn 1 (*sodium Salt*)
272. S 30c S 30c (fatty Acid)
273. Sna-2000 (*sodium Salt*)
274. Stearic Acid, Analytical Standard
275. Vlz 200
276. Stearic Acid High Purity 90% V
277. Purified Stearic Acid [nf]
278. Stearic Acid Flake 132 Nf Flake
279. Stearic Acid, Reagent Grade, 95%
280. Hy-b2219
281. Stearic Acid [ep Monograph]
282. Stearic Acid 400 (rubber Grade)
283. Zinc4978673
284. Tox21_111154
285. Tox21_201887
286. Tox21_300562
287. Bbl012224
288. Bdbm50240485
289. Lmfa01010018
290. S5733
291. Sa 200
292. Stearic Acid, >=95%, Fcc, Fg
293. Stl163565
294. Akos005716958
295. Tox21_111154_1
296. Ccg-267314
297. Db03193
298. Fa 1655
299. Fa 18:0
300. T16-55f
301. X33r8u0062
302. Ncgc00091596-01
303. Ncgc00091596-03
304. Ncgc00091596-04
305. Ncgc00091596-05
306. Ncgc00254456-01
307. Ncgc00259436-01
308. 68937-76-8
309. E570
310. Vs-03242
311. Stearic Acid, Puriss., >=98.5% (gc)
312. Stearic Acid, Saj First Grade, >=90.0%
313. Bb 0268543
314. Cs-0021598
315. Ft-0674650
316. Ft-0689088
317. G 270
318. S 300
319. S0163
320. Stearic Acid, Saj Special Grade, >=95.0%
321. Stearic Acid, Vetec(tm) Reagent Grade, 94%
322. 400jb9103-88
323. A 1760
324. C01530
325. D00119
326. Ec 250-178-0
327. F70008
328. Stearic Acid 50, Tested According To Ph.eur.
329. Vegetable Stearic Acid 7036 Fg, Kosher, Nf
330. Q209685
331. Sr-01000944717
332. Melting Point Standard 69-71c, Analytical Standard
333. Sr-01000944717-1
334. Stearic Acid, Grade I, >=98.5% (capillary Gc)
335. Stearic Acid, Saj First Grade, >=90.0%, Powder
336. F0001-1489
337. Stearic Acid (constituent Of Saw Palmetto) [dsc]
338. Stearic Acid, Certified Reference Material, Tracecert(r)
339. Z955123678
340. Stearic Acid (constituent Of Flax Seed Oil) [dsc]
341. Cd7993ea-ad14-452a-a907-33376cc98790
342. Stearic Acid, European Pharmacopoeia (ep) Reference Standard
343. Stearic Acid (constituent Of Evening Primrose Oil) [dsc]
344. Stearic Acid, United States Pharmacopeia (usp) Reference Standard
345. Stearic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 284.5 g/mol |
|---|---|
| Molecular Formula | C18H36O2 |
| XLogP3 | 7.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 16 |
| Exact Mass | 284.271530387 g/mol |
| Monoisotopic Mass | 284.271530387 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 202 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Stearic acid is known as a potent anti-inflammatory lipid. This fatty acid has profound and diverse effects on liver metabolism. The aim of this study was to investigate the effect of stearic acid on markers of hepatocyte transplantation in rats with acetaminophen (APAP)-induced liver damage. Wistar rats were randomly assigned to 10-day treatment. Stearic acid was administered to the rats with APAP-induced liver damage. The isolated liver cells were infused intraperitoneally into rats. Blood samples were obtained to evaluate the changes in the serum liver enzymes, including activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) and the level of serum albumin. To assess the engraftment of infused hepatocytes, rats were euthanized, and the liver DNA was used for PCR using sex-determining region Y (SRY) primers. The levels of AST, ALT and ALP in the serum of rats with APAP-induced liver injury were significantly increased and returned to the levels in control group by day six. The APAP-induced decrease in albumin was significantly improved in rats through cell therapy, when compared with that in the APAP-alone treated rats. SRY PCR analysis showed the presence of the transplanted cells in the liver of transplanted rats. Stearic acid-rich diet in combination with cell therapy accelerates the recovering of hepatic dysfunction in a rat model of liver injury.
PMID:27090202 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4983676 Hashemi Goradel N et al; Iran Biomed J 20 (4): 217-22 (2016)
/EXPL THER/ Because of their reported antiviral and anti-inflammatory activities, cream formulations containing n-docosanol (docosanol) or stearic acid were tested for effects on chemically-induced burns in mice. In this model, injury was induced by painting the abdomens of mice with a chloroform solution of phenol. This was followed by the topical application of test substances 0.5, 3, and 6 hr later. Progression of the wounds was assessed by a single evaluator after 8 hr, using a numerical score of gross morphology. Docosanol- and stearic acid-containing creams substantially and reproducibly lessened the severity and progression of skin lesions compared to untreated sites with a 76% and 57% reduction in mean lesion scores, respectively. Untreated wounds appeared red and ulcerated; docosanol cream-treated wounds showed only slight erythema.
PMID:10945745 Khalil MH et al; Contact Dermatitis 43 (2): 79-81 (2000)
LD100 Human oral 14286 mg/kg; practically nontoxic: probable oral lethal dose (human) more than 1 qt (2.2 lb) for 70 kg person (150 lb).
USEPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. HPV Data Set for Cobalt Stearate and Fatty Acids, Tall Oil, Cobalt Salts p.14 (2003)
A mild moisturizing body wash with stearic acid, a key component of corneum lipids, and emollient soybean oil has been introduced in the market place. The objectives of this study are to determine the amount and the location of the stearic acid in the corneum after in vivo cleansing by the formulation. Clinical cleansing studies for one and five consecutive days were carried out with the formulation containing soybean oil or petroleum jelly (PJ). The free stearic acid in it was replaced by the fully deuterated variant. The amounts of stearic acid in 10 consecutive corneum tape strips were measured by liquid chromatograph-mass spectroscopy. Separately, electron paramagnetic resonance (EPR) measurements were taken with a porcine skin after a wash by the soybean oil formulation with its free fatty acid replaced by its spin probe analogue, 5-doxyl stearic acid. Deuterated stearic acid was detected in all 10 consecutive layers of stratum corneum and the total amount after five washes with the soybean oil formulation was 0.33 ug/sq cm. The spin probe in cleanser-treated skin was incorporated in a partially ordered hydrophobic region similar to corneum lipids. The probe mobility increased in the temperature region where lipid disorder was expected. The estimated total fatty acid delivered to skin from cleansing is comparable to the amount of fatty acid in a corneum layer. The delivered fatty acid is most likely incorporated in the corneum lipid phase.
PMID:20883293 Mukherjee S et al; J Cosmet Dermatol 9 (3): 202-10 (2010)
It has been noted by several investigators that increasing fatty acid chain length slightly decreased their digestibility; stearic acid was the most poorly absorbed of the common fatty acids.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Fatty acids, /including stearic acid/, originating from adipose tissue stores are either bound to serum albumin or remain unesterified in the blood.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Oleic, palmitic, myristic, and stearic acids are primarily transported via the lymphatic system, and lauric acid is transported by the lymphatic and (as a free fatty acid) portal systems.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Radioactivity has been traced to the heart, liver, lung, spleen, kidney, muscle, intestine, adrenal, blood, and lymph, and adipose, mucosal, and dental tissues after administration of radioactive oleic, palmitic, and stearic acids.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Stearic acid metabolism via beta-oxidation, omega-oxidation, and (omega-1)-oxidation has been demonstrated in rat liver. Removal of a single acetate moiety can occur to produce palmitic acid, and both this and stearic acid may be desaturated, producing oleic and palmitoleic acids, respectively. After (l4)C stearic acid was injected into rats, about 50 percent of the liver (14)C was recovered as oleic acid, indicating that extensive desaturation occurs. Desaturation occurs only to a small extent extrahepatically but has been detected in adipose tissue and in cells of mammary tissue. Stearic acid is also incorporated into phospholipids, di- and triglycerides, cholesterol, cholesterol esters, and other sterol esters.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5 746
Proposed mechanisms for fatty acid uptake by different tissues range from passive diffusion to facilitated diffusion or a combination of both. Fatty acids taken up by the tissues can either be stored in the form of triglycerides (98% of which occurs in adipose tissue depots) or they can be oxidized for energy via the beta-oxidation and tricarboxylic acid cycle pathways of catabolism.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
The beta-oxidation of fatty acids occurs in most vertebrae tissues (except the brain) using an enzyme complex for the series of oxidation and hydration reactions resulting in the cleavage of acetate groups as acetyl-CoA (coenzyme A). An additional isomerization reaction is required for the complete catabolism of oleic acid. Alternate oxidation pathways can be found in the liver (omega-oxidation) and in the brain (alpha-oxidation).
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Fatty acid biosynthesis from acetyl-CoA takes place primarily in the liver, adipose tissue, and mammary glands of higher animals. Successive reduction and dehydration reactions yield saturated fatty acids up to a 16-carbon chain length. Stearic acid is synthesized by the condensation of palmitoyl-CoA and acetyl-CoA in the mitochondria, and oleic acid is formed via a mono-oxygenase system in the endoplasmic reticulum.
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p.341 Journal of the American College of Toxicology 6 (3): 321-401 (1987). Available from, as of November 15, 2017: https://www.cir-safety.org/ingredients
Animal cells can de novo synthesize palmitic and stearic fatty acid and their n-9 derivatives. However, de novo synthesis requires the utilization of energy. Palmitic acid (C16) is the immediate precursor of stearic acid (C18). In animal cells, oleic acid is created by the dehydrogenation (desaturation) of stearic acid. Oleic acid is further elongated and desaturated into a family of n-9 fatty acids. The demand for energy used to synthesize n-9 fatty acids can be reduced in cell culture by providing palmitic and stearic acids. In addition, since palmitic and stearic acid are saturated, they are not peroxidized during delivery to the cells.
Sigma-Aldrich; Stearic Acid in Cell Culture. Available from, as of November 22, 2017: https://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/stearic-acid.html
Stearic Acid has known human metabolites that include 17-Hydroxystearic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560


