



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
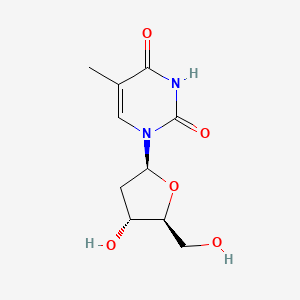

1. 1-(2-deoxy-beta-l-erythropentafuranosyl)-5-methyl-2,4(1h,3h)-pyrimidinedione
2. Beta L 2' Deoxythymidine
3. Beta-l-2'-deoxythymidine
4. Telbivudin
5. Tyzeka
1. 3424-98-4
2. 2'-deoxy-l-thymidine
3. Epavudine
4. L-thymidine
5. Beta-l-thymidine
6. Tyzeka
7. L-dt
8. Telbivudin
9. Sebivo
10. L-deoxythymidine
11. Nv-02b
12. Ldt600
13. Beta-l-2'-deoxythymidine
14. 26879-47-0
15. Ldt
16. Nv 02b
17. Ldt-600
18. Chebi:63624
19. 1-(2-deoxy-beta-l-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1h,3h)-dione
20. 2oc4hkd3sf
21. 1-((2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
22. 1-(2-deoxy-beta-l-ribofuranosyl)-5-methyluracil
23. Telbivudine [usan]
24. 1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione
25. Mfcd02683612
26. Llt
27. Tyzeka (tn)
28. Unii-2oc4hkd3sf
29. Telbivudine (usan/inn)
30. Telbivudine [usan:inn:ban]
31. 1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
32. Telbivudine [mi]
33. B-l-2'-deoxy-thymidine
34. Telbivudine [inn]
35. Telbivudine [vandf]
36. Telbivudine [mart.]
37. Telbivudine [who-dd]
38. Schembl124279
39. Telbivudine (sebivo, Tyzeka)
40. Telbivudine [ema Epar]
41. Chembl374731
42. Epavudine;l-thymidine;nv 02b
43. Thymine, 1-(2-deoxy-beta-l-erythro-pentofuranosyl)-
44. Zinc2159
45. Telbivudine, >=98% (hplc)
46. Dtxsid30187813
47. Telbivudine [orange Book]
48. Amy23971
49. Hy-b0017
50. Bdbm50088372
51. S1651
52. Akos025117349
53. Ac-5632
54. Ccg-266887
55. Cs-1934
56. Db01265
57. 2,4(1h,3h)-pyrimidinedione, 1-(2-deoxy-b-l-erythro-pentofuranosyl)-5-methyl-
58. 2,4(1h,3h)-pyrimidinedione, 1-(2-deoxy-beta-l-erythro-pentofuranosyl)-5-methyl-
59. Ncgc00346560-01
60. Ncgc00346560-06
61. Ncgc00346560-12
62. 1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione
63. As-35209
64. Sw220236-1
65. D06675
66. Ab01274717-01
67. Ab01274717_02
68. Q413621
69. Brd-k15976406-001-01-7
70. Thymine, 1-(2-deoxy-.beta.-l-erythro-pentofuranosyl)-
71. Z2574360267
72. 1-[(2r,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
73. 1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione
74. 2,4(1h,3h)-pyrimidinedione,1-(2-deoxy-.beta.-l-erythro-pentofuranosyl)-5-methyl-
75. 1-((2s,4s,5s)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
76. L-thymidine; Beta-l-thymidine; L-dt; 2'-deoxy-l-thymidine; 1-(2-deoxy-beta-l-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1h,3h)-dione
| Molecular Weight | 242.23 g/mol |
|---|---|
| Molecular Formula | C10H14N2O5 |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 242.09027155 g/mol |
| Monoisotopic Mass | 242.09027155 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 381 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Tyzeka |
| PubMed Health | Telbivudine (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | Tyzeka is the trade name for telbivudine, a synthetic thymidine nucleoside analogue with activity against hepatitisB virus (HBV). The chemical name for telbivudine is 1-((2S,4R,5S)-4-hydroxy-5-hydroxymethyltetrahydrofuran-2-y1)-5-methyl-1H-pyrimidi... |
| Active Ingredient | Telbivudine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 2 | |
|---|---|
| Drug Name | Tyzeka |
| PubMed Health | Telbivudine (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | Tyzeka is the trade name for telbivudine, a synthetic thymidine nucleoside analogue with activity against hepatitisB virus (HBV). The chemical name for telbivudine is 1-((2S,4R,5S)-4-hydroxy-5-hydroxymethyltetrahydrofuran-2-y1)-5-methyl-1H-pyrimidi... |
| Active Ingredient | Telbivudine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Novartis |
For the treatment of chronic hepatitis B in adult and adolescent patients 16 years of age with evidence of viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
Sebivo is indicated for the treatment of chronic hepatitis B in adult patients with compensated liver disease and evidence of viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis.
Initiation of Sebivo treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate.
Telbivudine is a synthetic thymidine nucleoside analogue with activity against hepatitis B virus (HBV). Telbivudine is the unmodified L enantiomer of the naturally occurring nucleoside, thymidine. It undergoes phosphorylation via interaction with cellular kinases to form the active metabolite, telbivudine 5'-triphosphate.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
J05AF11
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF11 - Telbivudine
Absorption
Absorbed following oral administration. Telbivudine absorption and exposure were unaffected when a single 600mg dose was administered with a highfat (~55 g), highcalorie (~950 kcal) meal.
Route of Elimination
Telbivudine is eliminated primarily by urinary excretion of unchanged drug.
Clearance
7.6 +/- 2.9 L/h [Normal renal function (Clcr>80 mL/min)]
5.0 +/- 1.2 L/h [Mild renal function impairement (Clcr=50-80 mL/min)]
2.6 +/- 1.2 L/h [Moderate renal function impairement (Clcr=30-49 mL/min)]
0.7 +/- 0.4 L/h [Severe renal function impairement (Clcr<30 mL/min)]
No metabolites of telbivudine were detected following administration of [14C]telbivudine in humans. Telbivudine is not a substrate, or inhibitor of the cytochrome P450 (CYP450) enzyme system.
Approximately 15 hours.
Telbivudine 5'triphosphate inhibits HBV DNA polymerase (reverse transcriptase) by competing with the natural substrate, thymidine 5'triphosphate. This leads to the chain termination of DNA synthesis, thereby inhibiting viral replication. Incorporation of telbivudine 5'triphosphate into viral DNA also causes DNA chain termination, resulting in inhibition of HBV replication. Telbivudine inhibits anticompliment or second-strand DNA.






