



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Td 6424
2. Td-6424
3. Td6424
4. Telavancin Hydrochloride
5. Vibativ
1. Telavancin [inn]
2. Televancin
3. 372151-71-8
4. Chebi:71229
5. Xk134822z0
6. Telavancina
7. Telavancine
8. Telavancinum
9. Unii-xk134822z0
10. Hsdb 8194
11. Telavancin [mi]
12. Telavancin [vandf]
13. Televancin [vandf]
14. Telavancin [mart.]
15. Telavancin [who-dd]
16. Chembl507870
17. Schembl8287015
18. Gtpl10925
19. Dtxsid10873383
20. Td6424
21. Db06402
22. Ac-30598
23. Hy-112959
24. Cs-0069761
25. T-1455
26. Telavancin, Antibiotic For Culture Media Use Only
27. Q7695658
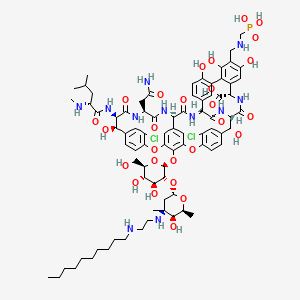
28. N(3'')-[2-(decylamino)ethyl]-29-{[(phosphonomethyl)amino]methyl}vancomycin
29. Vancomycin, N(sup 3'')-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-
30. Vancomycin, N3''-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-
| Molecular Weight | 1755.6 g/mol |
|---|---|
| Molecular Formula | C80H106Cl2N11O27P |
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 23 |
| Hydrogen Bond Acceptor Count | 31 |
| Rotatable Bond Count | 30 |
| Exact Mass | 1753.6374295 g/mol |
| Monoisotopic Mass | 1753.6374295 g/mol |
| Topological Polar Surface Area | 598 Ų |
| Heavy Atom Count | 121 |
| Formal Charge | 0 |
| Complexity | 3490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Vibativ |
| PubMed Health | Telavancin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | VIBATIV contains telavancin hydrochloride, a lipoglycopeptide antibacterial that is a synthetic derivative of vancomycin. The chemical name of telavancin hydrochloride is vancomycin,N3''-[2-(decylamino)ethyl]-29-[[(phosphono-methyl)-amino]-methy |
| Active Ingredient | Telavancin hydrochloride; Telavancin |
| Dosage Form | Powder; Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 750mg base/vial; 250mg; 750mg; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Theravance; Theravance Biopharma |
| 2 of 2 | |
|---|---|
| Drug Name | Vibativ |
| PubMed Health | Telavancin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | VIBATIV contains telavancin hydrochloride, a lipoglycopeptide antibacterial that is a synthetic derivative of vancomycin. The chemical name of telavancin hydrochloride is vancomycin,N3''-[2-(decylamino)ethyl]-29-[[(phosphono-methyl)-amino]-methy |
| Active Ingredient | Telavancin hydrochloride; Telavancin |
| Dosage Form | Powder; Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 750mg base/vial; 250mg; 750mg; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Theravance; Theravance Biopharma |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings. Telavancin. Online file (MeSH, 2014). Available from, as of May 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Vibativ is indicated for the treatment of adult patients with complicated skin and skin structure infections (cSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible and -resistant isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), or Enterococcus faecalis (vancomycin-susceptible isolates only). /Included in US product label/
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
Vibativ is indicated for the treatment of adult patients with hospital-acquired and ventilator associated bacterial pneumonia (HABP/VABP), caused by susceptible isolates of 45 Staphylococcus aureus (including methicillin-susceptible and -resistant isolates). Vibativ 46 should be reserved for use when alternative treatments are not suitable. /Included in US product label/
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
The US Food and Drug Administration (FDA) required and approved a Risk Evaluation and Mitigation Strategy (REMS) for telavancin. The goal of the telavancin REMS is to avoid unintended telavancin exposure in pregnant women by educating health-care providers and patients about the potential risk of fetal developmental toxicity and recommended measures to exclude and prevent pregnancy. The REMS requires that a telavancin medication guide be provided to the patient each time the drug is dispensed and outlines a communication plan requiring initial and periodic communications from the manufacturer to certain targeted groups of prescribers and pharmacists.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 438
To reduce development of drug-resistant bacteria and maintain effectiveness of telavancin and other antibacterials, the drug should be used only for treatment of infections proven or strongly suspected to be caused by susceptible bacteria. When selecting or modifying anti-infective therapy, results of culture and in vitro susceptibility testing should be used. In the absence of such data, local epidemiology and susceptibility patterns should be considered when selecting anti-infectives for empiric therapy. If documented or presumed pathogens include gram-negative or anaerobic bacteria, concomitant use of an anti-infective active against such bacteria may be clinically indicated.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 438
/BOXED WARNING/ WARNING: Patients with pre-existing moderate/severe renal impairment (CrCl .50 mL/min) who were treated with Vibativ for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia had increased mortality observed versus vancomycin. Use of Vibativ in patients with pre-existing moderate/severe renal impairment (CrCl .50 mL/min) should be considered only when the anticipated benefit to the patient outweighs the potential risk.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
/BOXED WARNING/ WARNING: Nephrotoxicity: New onset or worsening renal impairment has occurred. Monitor renal function in all patients.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
/BOXED WARNING/ WARNING: Women of childbearing potential should have a serum pregnancy test prior to administration of Vibativ. Avoid use of Vibativ during pregnancy unless potential benefit to the patient outweighs potential risk to the fetus. Adverse developmental outcomes observed in 3 animal species at clinically relevant doses raise concerns about potential adverse developmental outcomes in humans.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
Use of telavancin may result in overgrowth of nonsusceptible organisms, including fungi. The patient should be carefully monitored and appropriate therapy should be instituted if a superinfection occurs. Treatment with anti-infectives alters normal colon flora and may permit overgrowth of Clostridium difficile. C. difficile infection (CDI) and C. difficile-associated diarrhea and colitis (CDAD; also known as antibiotic-associated diarrhea and colitis or pseudomembranous colitis) have been reported with nearly all anti-infectives and may range in severity from mild diarrhea to fatal colitis. C. difficile produces toxins A and B which contribute to development of CDAD; hypertoxin-producing strains of C. difficile are associated with increased morbidity and mortality since these infections may be refractory to anti-infective therapy and may require colectomy. CDAD should be considered in the differential diagnosis of patients who develop diarrhea during or after anti-infective therapy. Careful medical history is necessary since CDAD has been reported to occur as late as 2 months or longer after anti-infective therapy is discontinued. If CDAD is suspected or confirmed, anti-infective therapy not directed against C. difficile should be discontinued whenever possible. Patients should be managed with appropriate supportive therapy (fluid and electrolyte management, protein supplementation), anti-infective therapy directed against C. difficile (e.g., metronidazole, vancomycin), and surgical evaluation as clinically indicated.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 438
For more Drug Warnings (Complete) data for Telavancin (18 total), please visit the HSDB record page.
For the treatment of complicated skin and skin structure infections (cSSSI) caused by gram-positive bacteria like methicillin-susceptible or -resistant Staphylococcus aureus, vancomycin-susceptible Enterococcus faecalis, and Streptococcus pyogenes, Streptococcus agalactiae, or Streptococcus anginosus group. Also for the treatment of adult patients with hospital-acquired bacterial pneumonia (HAP) and ventilator-associated bacterial pneumonia (VAP), known or suspected to be caused by susceptible isolates of Staphylococcus aureus (including methicillin-susceptible and methicillin-resistant S. aureus).
FDA Label
Vibativ is indicated for the treatment of adults with nosocomial pneumonia including ventilator-associated pneumonia, known or suspected to be caused by methicillin-resistant Staphylococcus aureus (MRSA).
Vibativ should be used only in situations where it is known or suspected that other alternatives are not suitable.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Telavancin is a semi-synthetic derivative of vancomycin, therefore the mode of bactericidal action is similar to vancomycin in which both antibiotics inhibit cell wall synthesis. Not only that, it displays concentration-dependent bactericidal action. Furthermore, telavancin is a more potent inhibitor (10-fold) of peptidoglycan synthesis and, unlike vancomycin, disrupts cell membrane integrity via its interaction with lipid II. AUC/MIC ratio best predicts the extent of in-vivo response in which the higher the ratio, the greater the bactericidal activity. The smallest ratio in which one would be able to observe no bacterial growth at 24 hours is 50. Maximal bactericidal activity is observed at a AUC/MIC ratio of 404.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XA03
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XA - Glycopeptide antibacterials
J01XA03 - Telavancin
Absorption
Telavancin demonstrates linear pharmacokinetics at doses between 1 and 12.5 mg/kg. Furthermore, 24 hours post-infusion of a dose of 7.5 to 15 mg/kg, activity against MRSA and penicillin-resistant Streptococcus pneumonia can still be observed. The trough concentration at this point of time is approximately 10 g/mL. Telavancin also has poor bioavailability and must be administered over 30-120 minutes IV. Cmax, healthy subjects, 10 mg/kg = 93.6 14.2 g/mL; AUC (0- ), healthy subjects, 10 mg/kg = 747 129 g h/mL; AUC (0-24h), healthy subjects, 10 mg/kg = 666 107 g h/mL; Time to steady state = 3 days;
Route of Elimination
Urine with >80% as unchanged drug and <20% as hydroxylated metabolites (with dose of 10mg/kg); Feces (<1%)
Volume of Distribution
Vss, healthy subjects, 10 mg/kg = 0.14 L/kg
Clearance
Cl, healthy subjects, 10 mg/kg = 13.9 2.9 mL/h/kg
Telavancin is primarily eliminated by the kidney. In a mass balance study, approximately 76% of the administered dose was recovered from urine and <1% of the dose was recovered from feces (collected up to 216 hours) based on total radioactivity.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
Telavancin binds to human plasma proteins, primarily to serum albumin, in a concentration-independent manner. The mean binding is approximately 90% and is not affected by renal or hepatic impairment. Concentrations of telavancin in skin blister fluid were 40% of those in plasma (AUC0-24hr ratio) after 3 daily doses of 7.5 mg/kg VIBATIV in healthy young adults.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
The mass balance and pharmacokinetics of telavancin, a semisynthetic lipoglycopeptide antimicrobial agent, were characterized in an open-label, phase 1 study of six healthy male subjects. After a single 1-h intravenous infusion of 10 mg/kg (14)C-telavancin (0.68 uCi/kg), blood, urine, and feces were collected at regular intervals up to 216 hr postdose. Whole blood, plasma, urine, and fecal samples were assayed for total radioactivity using scintillation counting; plasma and urine were also assayed for parent drug and metabolites using liquid chromatography with tandem mass spectrometry. The concentration-time profiles for telavancin and total radioactivity in plasma were comparable from 0 to 24 hr after the study drug administration. Telavancin accounted for >95% and 83% of total radioactivity in plasma at 12 hr and 24 hr, respectively. By 216 hr, approximately 76% of the total administered dose was recovered in urine while only 1% was collected in feces. Unchanged telavancin accounted for most (83%) of the eliminated dose. Telavancin metabolite THRX-651540 along with two other hydroxylated metabolites (designated M1 and M2) accounted for the remaining radioactivity recovered from urine. The mean concentrations of total radioactivity in whole blood were lower than the concentration observed in plasma, and mean concentrations of THRX-651540 in plasma were minimal relative to mean plasma telavancin concentrations. These observations demonstrate that most of an administered telavancin dose is eliminated unchanged via the kidneys. ...
PMID:20516282 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2916323 Shaw JP et al; Antimicrob Agents Chemother 54 (8): 3365-71 (2010)
The aim of this study was to assess the steady-state pharmacokinetic parameters of telavancin, an investigational bactericidal lipoglycopeptide, after intravenous (iv) administration to healthy male and female subjects. In a randomized, double-blind, parallel-group, gender-stratified, two-dose study, 79 adult subjects received three daily 60 min iv infusions of telavancin at 7.5 mg/kg (n = 40) or 15 mg/kg (n = 39). Blood and urine samples were collected for pharmacokinetic analyses at admission, on day 3 pre-infusion and up to 48 hr after the start of the day 3 infusion for 73 subjects (45 males and 28 females). Pharmacokinetic parameters were estimated by non-compartmental analysis. Following the day 3 telavancin dose (7.5 or 15 mg/kg), dose-proportional increases in mean peak plasma concentrations (C(max), 88 versus 186 mg/L for low and high doses, respectively) and total systemic exposures (AUC(0-24), 599 versus 1282 mg.h/L for low and high doses, respectively) were observed. Trough concentrations at steady state were 6 mg/L at 7.5 mg/kg/day and 16 mg/L at 15 mg/kg/day. The elimination half-life was dose-independent; the mean +/- SD ranged from 6.0 +/- 0.6 to 7.5 +/- 1.3 hr for low and high doses, respectively. Approximately two-thirds of the total telavancin dose was excreted unchanged in urine over 48 hr. Pharmacokinetic parameters were similar in males and females. Telavancin displayed linear plasma pharmacokinetics over the dose range 7.5-15 mg/kg/day and was primarily cleared via urinary excretion. No gender-related differences in the pharmacokinetic disposition of telavancin were observed. These data further characterize the pharmacokinetic profile of telavancin, a once-daily therapy targeted for the treatment of serious Gram-positive infections.
PMID:18586659 Wong SL et al; J Antimicrob Chemother 62 (4): 780-3 (2008)
For more Absorption, Distribution and Excretion (Complete) data for Telavancin (8 total), please visit the HSDB record page.
Metabolism of telavancin does not involve the cytochrome P450 enzyme system. Primary metabolite is called THRX-651540, but the metabolite pathway has not been identified.
In a mass balance study in male subjects using radiolabeled telavancin, 3 hydroxylated metabolites were identified with the predominant metabolite (THRX-651540) accounting for <10% of the radioactivity in urine and <2% of the radioactivity in plasma. The metabolic pathway for telavancin has not been identified.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
No metabolites of telavancin were detected in in vitro studies using human liver microsomes, liver slices, hepatocytes, and kidney S9 fraction. None of the following recombinant CYP 450 isoforms were shown to metabolize telavancin in human liver microsomes: CYP 1A2, 2C9, 2C19, 2D6, 3A4, 3A5, 4A11.
NIH; DailyMed. Current Medication Information for Vibativ (Telavancin Hydrochloride) Injection, Powder, Lyophilized, For Solution (Revised: March 2014). Available from, as of June 11, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=9fc67b76-9ba1-45ec-9749-ca3f3a6477d9
Telavancin was not extensively metabolized in rats, dogs and monkeys after IV administration. Unchanged telavancin was the predominant component in the serum (99, 89 and 94 % of total AUC for rats, dogs and monkeys, respectively) while 7-OH-telavancin (AMI-11352), telavancin des-phosphonate (AMI-999) and other OH-metabolites were identified. Telavancin accounted for more than 60% (dogs) and 86% (monkeys) of the urinary recoveries. AMI-11352 represented about 17% (dogs) and 5% (monkeys) of total urinary recovery while AMI-999 represented about 1.2% (dogs) and 1.8% (monkeys) and other OH-metabolites represented about 17% (dogs) and 6% (monkey). There was no significant gender-related difference observed for metabolism profiles. Of the three OH-metabolites of the 2-(decylamino) ethyl side chain of telavancin identified in human urine 7-OH-telavancin (AMI-11352) was the most abundant. The plasma AUC of 7-OH-telavancin (which is much less active against bacteria than telavancin) was about 2-3% of the AUC of telavancin and accounted for 50% of total peak areas of the three hydroxylated metabolites. AMI-11355 (8-OH metabolite) and AMI-11353 (9-OH metabolite) accounted for 24.2% and 25.3% of the total peak areas of the three hydroxylated metabolites, respectively. Plasma concentrations of AMI-11352 were low in the rat and increases in Cmax and AUC0-24 were less than dose-proportional. Systemic exposure to AMI-11352 was larger in dogs compared to rats. According to the applicant, saturation of the metabolic pathway at higher doses may be anticipated as the AUC0-t metabolite/telavancin ratio decreased at high doses in both rats and dogs. Systemic exposures to telavancin, AMI-999 and AMI-11352 in rats and/or dogs at steady state exceeded human systemic exposure at the proposed clinical dose of 10 mg/kg/day.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Vibativ (Telavancin) p.17 (2011).
The main metabolite of telavancin, 7-OH-Telavancin (AMI-11352), has antibacterial activity but is 10-fold less potent than telavancin. Due to the low antibacterial activity of AMI-11352 and the low human exposure, this metabolite is not considered to have a relevant contribution to the overall activity of telavancin in vivo.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Vibativ (Telavancin) p.14 (2011). Available from, as of May 29, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001240/WC500115363.pdf
Terminal elimination half-life = 8 1.5 hours (with normal renal function)
Following single doses of 10 mg/kg telavancin (IV bolus injection or infusion) the serum or plasma concentrations of telavancin declined in all species with half life ranging from 1.2 hours in mice to 2.3 hours in monkeys.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Vibativ (Telavancin) p.15 (2011). Available from, as of May 29, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001240/WC500115363.pdf
In a randomized, double-blind, parallel-group, gender-stratified, two-dose study, 79 adult subjects received three daily 60 min iv infusions of telavancin at 7.5 mg/kg (n = 40) or 15 mg/kg (n = 39). Blood and urine samples were collected for pharmacokinetic analyses at admission, on day 3 pre-infusion and up to 48 hr after the start of the day 3 infusion for 73 subjects (45 males and 28 females). ... The elimination half-life was dose-independent; the mean +/- SD ranged from 6.0 +/- 0.6 to 7.5 +/- 1.3 hr for low and high doses, respectively. ...
PMID:18586659 Wong SL et al; J Antimicrob Chemother 62 (4): 780-3 (2008)
In the pigmented rat quantitative whole body autoradiography (QWBA) study the highest levels of radioactivity at 168 hours post dose were observed in liver, spleen and kidney and at 336 hours post dose were observed in spleen, adrenal gland and kidney. The half-life estimated for liver and kidney were 4 and 5 days, respectively. The high levels of radioactivity observed in the bone at all time points appeared to be mainly located in the growth plates and also in the bone marrow with an estimated half-life of 332 hours (approximately 14 days). Penetration into the CNS was minimal.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Vibativ (Telavancin) p.17 (2011). Available from, as of May 29, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001240/WC500115363.pdf
Telavancin is a bactericidal lipoglycopeptide that is active against a broad range of gram-positive bacteria. Telavancin prevents polymerization of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) and cross-linking of peptidoglycan by binding to D-Ala-D-Ala. As a result, inhibition of bacterial cell wall synthesis occurs. Furthermore, telavancin disrupts membrane potential and cell permeability as a result of the lipophillic side chain moiety. This additional bactericidal mechanism is what sets telavancin apart from vancomycin.
The emergence and spread of multidrug-resistant gram-positive bacteria represent a serious clinical problem. Telavancin is a novel lipoglycopeptide antibiotic that possesses rapid in vitro bactericidal activity against a broad spectrum of clinically relevant gram-positive pathogens. ... As observed with vancomycin, telavancin inhibited late-stage peptidoglycan biosynthesis in a substrate-dependent fashion and bound the cell wall, as it did the lipid II surrogate tripeptide N,N'-diacetyl-L-lysinyl-D-alanyl-D-alanine, with high affinity. Telavancin also perturbed bacterial cell membrane potential and permeability. In methicillin-resistant Staphylococcus aureus, telavancin caused rapid, concentration-dependent depolarization of the plasma membrane, increases in permeability, and leakage of cellular ATP and K(+). The timing of these changes correlated with rapid , concentration-dependent loss of bacterial viability, suggesting that the early bactericidal activity of telavancin results from dissipation of cell membrane potential and an increase in membrane permeability. Binding and cell fractionation studies provided direct evidence for an interaction of telavancin with the bacterial cell membrane; stronger binding interactions were observed with the bacterial cell wall and cell membrane relative to vancomycin.
PMID:15728913 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC549257 Higgins DL et al; Antimicrob Agents Chemother 49 (3): 1127-34 (2005)
Telavancin usually is bactericidal in action. Telavancin inhibits bacterial cell wall synthesis by inhibiting peptidoglycan synthesis and blocking the transglycosylation step. Telavancin binds to the bacterial membrane and disrupts membrane barrier function.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 439
Telavancin is a novel semisynthetic lipoglycopeptide derivative of vancomycin with a decylaminoethyl side chain that is active against Gram-positive bacteria, including Staphylococcus aureus strains resistant to methicillin or vancomycin. A dual mechanism of action has been proposed for telavancin involving inhibition of peptidoglycan biosynthesis and membrane depolarization. ... The results of genome-wide transcriptional profiling of the response of S. aureus to telavancin using microarrays /is reported/. Short (15-min) challenge of S. aureus with telavancin revealed strong expression of the cell wall stress stimulon, a characteristic response to inhibition of cell wall biosynthesis. In the transcriptome obtained after 60-min telavancin challenge, in addition to induction of the cell wall stress stimulon, there was induction of various genes, including lrgA and lrgB, lysine biosynthesis operon (dap) genes, vraD and vraE, and hlgC, that have been reported to be induced by known membrane-depolarizing and active agents, including carbonyl cyanide m-chlorophenylhydrazone, daptomycin, bacitracin, and other antimicrobial peptides These genes were either not induced or only weakly induced by the parent molecule vancomycin. /It is suggested/ that expression of these genes is a response of the cell to mitigate and detoxify such molecules and is diagnostic of a membrane-depolarizing or membrane-active molecule. The results indicate that telavancin causes early and significant induction of the cell wall stress stimulon due to strong inhibition of peptidoglycan biosynthesis, with evidence in support of membrane depolarization and membrane activity that is expressed after a longer duration of drug treatment.
PMID:22411615 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3370745 Song Y et al; Antimicrob Agents Chemother 56 (6): 3157-64 (2012)
The cellular binding patterns of fluorescent conjugates of telavancin and vancomycin were evaluated in Staphylococcus aureus by fluorescence microscopy and ratio imaging analysis. Telavancin showed enhanced binding at the division septum compared to vancomycin. This result is consistent with observations that telavancin binds with higher affinity to lipid II than to d-Ala-d-Ala residues in the cell wall, thus demonstrating the preferential binding of telavancin to the site of active cell wall biosynthesis.
PMID:20176907 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2863671 Lunde CS et al; Antimicrob Agents Chemother 54 (5): 2198-200 (2010)


