
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Australia
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
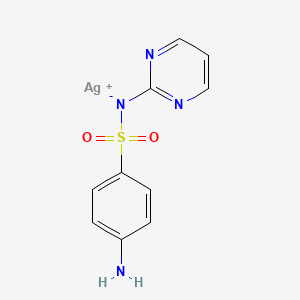

1. Brandiazin
2. Dermazin
3. Flamazine
4. Flammazine
5. Sicazine
6. Silvadene
7. Silvederma
8. Silver Sulfafdiazine
9. Ssd
10. Ssd Af
11. Sulfadiazine, Silver
12. Sulfafdiazine, Silver
13. Sulfargen
14. Thermazene
1. 22199-08-2
2. Sulfadiazine Silver
3. Flamazine
4. Sulfadiazine Silver Salt
5. Silvadene
6. Dermazin
7. Silver Sulphadiazine
8. Thermazene
9. Sulfadiazine, Silver
10. Dermazine
11. Geben
12. Silver Sulfadiazinate
13. Silvazine
14. Altreet-ag
15. Mfcd00072101
16. Ssd
17. W46jy43ejr
18. Chebi:9142
19. Benzenesulfonamide, 4-amino-n-2-pyrimidinyl-, Monosilver(1+) Salt
20. Silver;(4-aminophenyl)sulfonyl-pyrimidin-2-ylazanide
21. N(sup 1)-2-pyrimidinylsulfanilamide Monosilver(1+) Salt
22. Silbertone
23. Silver(i) Sulfadiazine
24. Sulfadiazin Silber
25. Dsstox_cid_28572
26. Dsstox_rid_82844
27. Dsstox_gsid_48646
28. Sulfadiazin, Silbersalz
29. Silvadene (tn)
30. Silver(1+) [(4-aminophenyl)sulfonyl](pyrimidin-2-yl)azanide
31. Sulfadiazine, Silver [usan]
32. Cas-22199-08-2
33. Nsc625324
34. Ncgc00183137-01
35. Ncgc00188438-01
36. Unii-w46jy43ejr
37. N1-2-pyrimidinylsulfanilamide Monosilver(1+) Salt
38. Sulfadiazine, Silver (usp)
39. 4-amino-n-(2-pyrimidinyl)benzenesulfonamide Silver Salt
40. Sulfadiazine, Silver [usan:usp]
41. Einecs 244-834-5
42. Sulfadiazinum Argentum
43. Nsc 625324
44. (4-amino-n-pyrimidin-2-ylbenzenesulphonamidato-nn,o1)silver
45. Silver Sulfadiazine Usp
46. Silver, (4-amino-n-(2-pyrimidinyl-.kappa.n1)benzenesulfonamidato-.kappa.o)-
47. Silver, (4-amino-n-2-pyrimidinylbenzenesulfonamidato-nn,o1)-
48. Silver (i) Sulfadiazine
49. Schembl15633
50. Sulfadiazine Silver (jp17)
51. Silver Sulfadiazine Bulk Powder
52. Chembl1382627
53. Dtxsid4048646
54. Sulfadiazine Silver [jan]
55. (4-amino-n-2-pyrimidinylbenzenesulfonamidato-nn,01)-silver
56. Sulfanilamide, N(sup 1)-2-pyrimidinyl-, Monosilver(1+) Salt
57. Silver Sulfadiazine [vandf]
58. Sulfadiazine Silver [mart.]
59. Tox21_112998
60. Tox21_113471
61. Silver Sulfadiazine [usp-rs]
62. Sulfadiazine Silver [who-dd]
63. Sulfadiazine Silver [who-ip]
64. Sulfadiazine Silver Salt [mi]
65. Akos005111099
66. Ac-3535
67. Db05245
68. Silver Sulfadiazine [green Book]
69. Silver Sulfadiazine [orange Book]
70. As-13863
71. Sy105573
72. Silver Sulfadiazine [usp Monograph]
73. Db-045839
74. Sulfadiazine, Silver [usp Impurity]
75. Ft-0630481
76. S0595
77. Sulfadiazinum Argentum [who-ip Latin]
78. D00433
79. Q420984
80. J-014582
81. Silver (4-aminophenyl)sulfonyl-pyrimidin-2-yl-azanide
82. [(4-aminophenyl)sulfonyl](pyrimidin-2-yl)azanide (ag+)
83. 4-amino-n-(2-pyrimidinyl) Benzenesulfonamide Silver Salt
84. Silver(i) ((4-aminophenyl)sulfonyl)(pyrimidin-2-yl)amide
85. Silver(1+) Ion 4-{[(pyrimidin-2-yl)azanidyl]sulfonyl}aniline
86. Benzenesulfonamide, 4-amino-n-2-pyrimidinyl-, Silver(1+) Salt (1:1)
87. 1152234-18-8
1. Soludiazine
2. Suthogen
3. Sulfadiazin-natrium
4. Sulfadiazine Silver
| Molecular Weight | 357.14 g/mol |
|---|---|
| Molecular Formula | C10H9AgN4O2S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 355.94971 g/mol |
| Monoisotopic Mass | 355.94971 g/mol |
| Topological Polar Surface Area | 95.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Silvadene |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | King Pharms |
| 2 of 8 | |
|---|---|
| Drug Name | Ssd |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | SILVADENE Cream 1% is a soft, white, water-miscible cream containing the antimicrobial agent silver sulfadiazine in micronized form, which has the following structural formula: Each gram of SILVADENE Cream 1% contains 10 mg of micronized silver sulfa... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 3 of 8 | |
|---|---|
| Drug Name | Ssd af |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | SSD (1% Silver Sulfadiazine Cream) and SSD AF (1% Silver Sulfadiazine Cream), 1% are topical antibacterial preparations which have as their active antimicrobial ingredient silver sulfadiazine. The active moiety is contained within an opaque, wh... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 4 of 8 | |
|---|---|
| Drug Name | Thermazene |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Thermazene (silver sulfadiazine Cream, 1% is a soft, water dispersible cream containing silver sulfadiazine in micronized form for topical application. Each gram of Thermazene contains 10mg of micronized silver sulfadiazine. Thermazene contains 1... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Thepharmanetwork |
| 5 of 8 | |
|---|---|
| Drug Name | Silvadene |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | King Pharms |
| 6 of 8 | |
|---|---|
| Drug Name | Ssd |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | SILVADENE Cream 1% is a soft, white, water-miscible cream containing the antimicrobial agent silver sulfadiazine in micronized form, which has the following structural formula: Each gram of SILVADENE Cream 1% contains 10 mg of micronized silver sulfa... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 7 of 8 | |
|---|---|
| Drug Name | Ssd af |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | SSD (1% Silver Sulfadiazine Cream) and SSD AF (1% Silver Sulfadiazine Cream), 1% are topical antibacterial preparations which have as their active antimicrobial ingredient silver sulfadiazine. The active moiety is contained within an opaque, wh... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 8 of 8 | |
|---|---|
| Drug Name | Thermazene |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Thermazene (silver sulfadiazine Cream, 1% is a soft, water dispersible cream containing silver sulfadiazine in micronized form for topical application. Each gram of Thermazene contains 10mg of micronized silver sulfadiazine. Thermazene contains 1... |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Thepharmanetwork |
Indicated as an adjunct for the prevention and treatment of wound sepsis in patients with second- and third-degree burns.
Silver sulfadiazine has broad antimicrobial activity. It is bactericidal for many gram- negative and gram-positive bacteria as well as being effective against yeast. Silver sulfadiazine is not a carbonic anhydrase inhibitor and may be useful in situations where such agents are contraindicated.
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D06BA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BA - Sulfonamides
D06BA01 - Silver sulfadiazine
Absorption
Very limited penetration through the skin. Only when applied to very large area burns is absorption into the body generally an issue.
Studies utilizing radioactive micronized silver sulfadiazine, electron microscopy, and biochemical techniques have revealed that the mechanism of action of silver sulfadiazine on bacteria differs from silver nitrate and sodium sulfadiazine. Silver sulfadiazine acts only on the cell membrane and cell wall to produce its bactericidal effect. A specific mechanism of action has not been determined, but silver sulfadiazine's effectiveness may possibly be from a synergistic interaction, or the action of each component. Silver is a biocide, which binds to a broad range of targets. Silver ions bind to nucleophilic amino acids, as well as sulfhydryl, amino, imidazole, phosphate, and carboxyl groups in proteins, causing protein denaturation and enzyme inhibition. Silver binds to surface membranes and proteins, causing proton leaks in the membrane, leading to cell death. Sulfadiazine is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
 Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.

| Available Reg Filing : EDQM Certified Facility |
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Bioquim: An European GMP-certified company manufacturing bulk APIs, specializing in sterile lyophilization and chemical synthesis.
Bioquim: An European GMP-certified company manufacturing bulk APIs, specializing in sterile lyophilization and chemical synthesis.
 Macsen Labs, a leader in Chemistry since 1952, specializing in APIs, specialty and fine chemicals, and dyes.
Macsen Labs, a leader in Chemistry since 1952, specializing in APIs, specialty and fine chemicals, and dyes.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33052
Submission : 2018-08-08
Status : Active
Type : II

Date of Issue : 2025-10-15
Valid Till : 2028-10-14
Written Confirmation Number : WC-0438
Address of the Firm :

NDC Package Code : 73379-002
Start Marketing Date : 2020-03-18
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN PRESCRIPTION COMPOUNDING
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
 JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry
JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry

Date of Issue : 2025-07-04
Valid Till : 2027-12-16
Written Confirmation Number : WC-0336
Address of the Firm :

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22083
Submission : 2008-04-03
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4297
Submission : 1981-09-29
Status : Active
Type : II



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29229
Submission : 2015-03-26
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
40
PharmaCompass offers a list of Silver Sulfadiazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Silver Sulfadiazine manufacturer or Silver Sulfadiazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Silver Sulfadiazine manufacturer or Silver Sulfadiazine supplier.
PharmaCompass also assists you with knowing the Silver Sulfadiazine API Price utilized in the formulation of products. Silver Sulfadiazine API Price is not always fixed or binding as the Silver Sulfadiazine Price is obtained through a variety of data sources. The Silver Sulfadiazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sulfadiazine Silver manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sulfadiazine Silver, including repackagers and relabelers. The FDA regulates Sulfadiazine Silver manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sulfadiazine Silver API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sulfadiazine Silver manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sulfadiazine Silver supplier is an individual or a company that provides Sulfadiazine Silver active pharmaceutical ingredient (API) or Sulfadiazine Silver finished formulations upon request. The Sulfadiazine Silver suppliers may include Sulfadiazine Silver API manufacturers, exporters, distributors and traders.
click here to find a list of Sulfadiazine Silver suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sulfadiazine Silver DMF (Drug Master File) is a document detailing the whole manufacturing process of Sulfadiazine Silver active pharmaceutical ingredient (API) in detail. Different forms of Sulfadiazine Silver DMFs exist exist since differing nations have different regulations, such as Sulfadiazine Silver USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sulfadiazine Silver DMF submitted to regulatory agencies in the US is known as a USDMF. Sulfadiazine Silver USDMF includes data on Sulfadiazine Silver's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sulfadiazine Silver USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sulfadiazine Silver suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sulfadiazine Silver Drug Master File in Japan (Sulfadiazine Silver JDMF) empowers Sulfadiazine Silver API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sulfadiazine Silver JDMF during the approval evaluation for pharmaceutical products. At the time of Sulfadiazine Silver JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sulfadiazine Silver suppliers with JDMF on PharmaCompass.
A Sulfadiazine Silver written confirmation (Sulfadiazine Silver WC) is an official document issued by a regulatory agency to a Sulfadiazine Silver manufacturer, verifying that the manufacturing facility of a Sulfadiazine Silver active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sulfadiazine Silver APIs or Sulfadiazine Silver finished pharmaceutical products to another nation, regulatory agencies frequently require a Sulfadiazine Silver WC (written confirmation) as part of the regulatory process.
click here to find a list of Sulfadiazine Silver suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sulfadiazine Silver as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sulfadiazine Silver API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sulfadiazine Silver as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sulfadiazine Silver and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sulfadiazine Silver NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sulfadiazine Silver suppliers with NDC on PharmaCompass.
Sulfadiazine Silver Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sulfadiazine Silver GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sulfadiazine Silver GMP manufacturer or Sulfadiazine Silver GMP API supplier for your needs.
A Sulfadiazine Silver CoA (Certificate of Analysis) is a formal document that attests to Sulfadiazine Silver's compliance with Sulfadiazine Silver specifications and serves as a tool for batch-level quality control.
Sulfadiazine Silver CoA mostly includes findings from lab analyses of a specific batch. For each Sulfadiazine Silver CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sulfadiazine Silver may be tested according to a variety of international standards, such as European Pharmacopoeia (Sulfadiazine Silver EP), Sulfadiazine Silver JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sulfadiazine Silver USP).

