



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
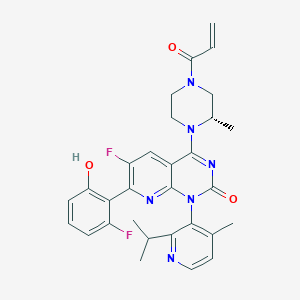

1. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2s)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one
2. Amg 510
3. Amg-510
4. Amg510
5. Lumakras
1. Amg-510
2. Amg510
3. 2296729-00-3
4. Lumakras
5. Amg-510 Racemate
6. Amg 510
7. 2252403-56-6
8. Kras G12c Inhibitor 9
9. Sotorasib [inn]
10. Sotorasib [usan]
11. Kras Mutant-targeting Amg 510
12. 4-((s)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1h)-one
13. 2b2vm6uc8g
14. Chembl4535757
15. 2296729-00-3 (racemate)
16. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2s)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one
17. (1m)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2s)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1h)-one
18. (1s)-4-((s)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1h)-one
19. 2296729-66-1
20. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2s)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one
21. Pyrido(2,3-d)pyrimidin-2(1h)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2s)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
22. Sotorasibum
23. Lumykras
24. Pyrido(2,3-d)pyrimidin-2(1h)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2s)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-, (1r)-
25. Pyrido[2,3-d]pyrimidin-2(1h)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2s)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]-, (1r)-
26. Amg510 Racemate
27. Amg 510 Racemate
28. Amg-510(racemate)
29. Sotorasib [jan]
30. Sotorasib Racemate
31. Unii-2b2vm6uc8g
32. Sotorasib [who-dd]
33. Sotorasib [orange Book]
34. Schembl20560375
35. Gtpl10678
36. Chebi:178199
37. Amg 510 Pound>>amg-510
38. Dtxsid001099260
39. Glxc-25372
40. Amy16918
41. Bcp30452
42. Bcp33368
43. Ex-a3538
44. Bdbm50514402
45. Nsc818433
46. S8830
47. Who 11370
48. Akos037649138
49. Db15569
50. Nsc-818433
51. Ac-35168
52. Ba172505
53. Ba172506
54. Bs-16684
55. Hy-114277
56. Cs-0081316
57. Compound (r)-38 [pmid: 31820981]
58. D70074
59. D77975
60. A930071
61. A934531
62. Amg510 ; Amg 510; Amg-510; Amg510
63. (1r)-4-((s)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1h)-one
64. (1ra)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-4-((2s)-2-methyl-4-(1-oxoprop-2-en-1-yl)piperazin-1-yl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)pyrido(2,3-d)pyrimidin-2(1h)-one
65. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-(1m)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2s)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1h)-one
66. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(2-propanyl)-3-pyridinyl]-4-[(2s)-2-methyl-4-(2-propenoyl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1h)-one
67. 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2s)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl]pyrido[2,3-d]pyrimidin-2(1h)-one
| Molecular Weight | 560.6 g/mol |
|---|---|
| Molecular Formula | C30H30F2N6O3 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 560.23474516 g/mol |
| Monoisotopic Mass | 560.23474516 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1030 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sotorasib is indicated in the treatment of adults with KRAS G12C mutant non small cell lung cancer.
Lumykras as monotherapy is indicated for the treatment of adults with advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutation and who have progressed after at least one prior line of systemic therapy.
Sotorasib is indicated in the treatment of adults with KRAS G12C mutant non small cell lung cancer. It has a moderate duration of action as it is given daily. Patients should be counselled regarding the risks of hepatotoxicity, interstitial lung disease and pneumonitis; and to avoid breastfeeding during treatment and up to 1 week after the last dose.
Immune Checkpoint Inhibitors
Drugs that block negative regulator IMMUNE CHECKPOINT proteins (e.g., PD-1 RECEPTOR and CTLA-4 ANTIGEN) thereby increasing suppressed immune activation in immunotherapies. (See all compounds classified as Immune Checkpoint Inhibitors.)
L01XX73
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX73 - Sotorasib
Absorption
A 960 mg once daily dose of sotorasib reaches a Cmax of 7.50 g/mL, with a median Tmax of 2.0 hours, and an AUC0-24h of 65.3 h\*g/mL.
Route of Elimination
Sotorasib is 74% eliminated in the feces and 6% eliminated in the urine. 53% of the dose recovered in the feces and 1% of the dose recovered in the urine is in the form of the unchanged parent compound.
Volume of Distribution
The volume of distribution of sotorasib is 211 L.
Clearance
Sotorasib has an apparent clearance at steady state of 26.2 L/h.
Sotorasib is predominantly metabolized through conjugation or by CYP3As.
Sotorasib has a terminal elimination half life of 5.5 1.8 hours.
Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway. GTP is hydrolyzed to GDP, and KRAS is inactivated. KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. Sotorasib binds to the cysteine residue in KRAS G12C mutations, holding the protein in its inactive form. The cysteine residue that sotorasib targets is not present in the wild type KRAS, which prevents off-target effects. This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors.


