


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Aluminum Monostearate
2. Aluminum Tristearate
3. Ammonium Stearate
4. Calcium Stearate
5. Magnesium Stearate
6. Octadecanoic Acid
7. Stearic Acid
8. Zinc Stearate
1. 822-16-2
2. Sodium Octadecanoate
3. Octadecanoic Acid, Sodium Salt
4. Flexichem B
5. Stearates
6. Sodiumstearate
7. Stearic Acid, Sodium Salt
8. Sodium;octadecanoate
9. Prodhygine
10. Bonderlube 235
11. Edenor Fhti
12. Nonsoul Sn 15
13. Sodium Stearate [nf]
14. Qu7e2xa9tg
15. Rhenogran Nast 50acmf-ge1858
16. Sodium Stearate (nf)
17. Stearic Acid Sodium Salt
18. Sodium Stearate, Pure
19. Hsdb 5759
20. Einecs 212-490-5
21. Unii-qu7e2xa9tg
22. Mfcd00036404
23. Ai3-19808
24. Sodium Palmitostearate
25. Octadecanoic Acid, Sodium Salt (1:1)
26. Prifer 1634
27. Rashayan Sodium Stearate
28. Schembl5773
29. Sodium Stearate [ii]
30. Sodium Stearate [mi]
31. Sodium Stearate [hsdb]
32. Sodium Stearate [inci]
33. Sodium Stearate (a Mixture Of Stearate And Palmitate)
34. Sodium Stearate [vandf]
35. Chembl1906423
36. Dtxsid9027318
37. Sodium Stearate [mart.]
38. Sodium Stearate [who-dd]
39. Chebi:132109
40. Akos028109686
41. Ncgc00164255-01
42. As-15926
43. E-470(i)stearic Acid, Sodium Salt
44. Cs-0152212
45. Ins-470(i)stearic Acid, Sodium Salt
46. D05875
47. D92227
48. Ins No.470(i)stearic Acid, Sodium Salt
49. A806549
50. A840275
51. Q420066
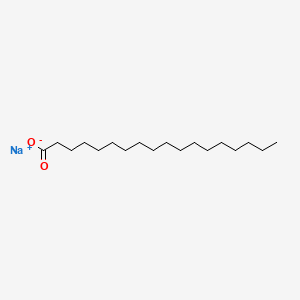
| Molecular Weight | 306.5 g/mol |
|---|---|
| Molecular Formula | C18H35NaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 16 |
| Exact Mass | 306.25347464 g/mol |
| Monoisotopic Mass | 306.25347464 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 207 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
SEE SOAPS: 2. 2= SLIGHTLY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-15 G/KG, BETWEEN 1 PINT & 1 QUART FOR 70 KG PERSON (150 LB). /SOAPS/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-179


