



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
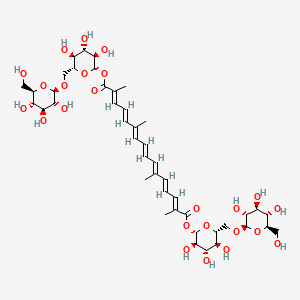

1. Alpha-crocin
1. Gardenia Yellow
2. Crocin I
3. Alpha-crocin
4. 42553-65-1
5. 94238-00-3
6. Crocine
7. Saffron
8. Crocin 1
9. .alpha.-crocin
10. Crocin-1
11. Crocetin Digentiobiose Ester
12. Bis[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl] (2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
13. Crocetin Di-gentiobiose Ester
14. Chembl446785
15. Chebi:79068
16. 877gwi46c2
17. 11012-59-2
18. Crocetin Bis(gentiobiosyl) Ester
19. Ncgc00160471-01
20. Bis(6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl) 8,8'-diapo-psi,psi-carotenedioate
21. All-trans-crocetin Di-beta-d-gentiobiosyl Ester
22. Crocin A
23. Ccris 678
24. Ccris 7705
25. Crocetin Digentiobiosyl Ester
26. Einecs 255-881-6
27. Brn 6473367
28. Unii-f32ba2h92z
29. Unii-877gwi46c2
30. Crocin-i
31. Hsdb 8211
32. Natural Red 1
33. Einecs 254-465-1
34. Natural Yellow 19
35. Crocetin Digentiobioside
36. Crocin [inci]
37. Crocin [who-dd]
38. Trans-crocetin Di(beta-d-gentiobiosyl) Ester
39. Dsstox_cid_1457
40. Dsstox_rid_81403
41. Dsstox_gsid_46172
42. F32ba2h92z
43. Schembl1463936
44. Crocin (gardenia Fruit Extract)
45. Dtxsid7046172
46. Bis(beta-d-gentiobiosyl) Crocetin
47. Hms3887o07
48. Crocetin Di(beta-gentiobiosyl)ester
49. Hy-n0697
50. Crocetin Di-beta-d-gentiobiose Ester
51. Tox21_111837
52. Bdbm50260195
53. Mfcd11044877
54. S9511
55. Akos015896765
56. Zinc245224178
57. Db11874
58. Ncgc00160471-02
59. 8,8'-diapo-.psi.,.psi.-carotenedioic Acid, Bis(6-o-.beta.-d-glucopyranosyl-.beta.-d-glucopyranosyl) Ester
60. 8,8'-diapo-psi,psi-carotenedioic Acid, Bis(6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl) Ester
61. Beta-d-glucopyranose, 6-o-beta-d-glucopyranosyl-, 1,1'-((2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethyl-2,4,6,8,10,12,14-hexadecaheptaenedioate)
62. Crocetin Di-gentiobiose Ester [mi]
63. Cas-42553-65-1
64. C.i. 75100
65. Cs-0009714
66. N1653
67. N1661
68. N1889
69. Trans-crocetin Bis(beta-d-gentiobiosyl) Ester
70. C08589
71. A872860
72. Q424767
73. Trans-crocetin Di(.beta.-d-gentiobiosyl) Ester
74. All-trans-crocetin Di-.beta.-d-gentiobiosyl Ester
75. (2e,4e,6e,8e,10e,12e,14e)-bis((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)methyl)tetrahydro-2h-pyran-2-yl) 2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
76. (2e,4e,6e,8e,10e,12e,14e)-bis((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)methyl)tetrahydro-2h-pyran-2-yl)2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
77. .beta.-d-glucopyranose, 6-o-.beta.-d-glucopyranosyl-, 1,1'-((2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethyl-2,4,6,8,10,12,14-hexadecaheptaenedioate)
78. Bis(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-({[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl (2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
79. Bis[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-({[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl]oxy}methyl)tetrahydro-2h-pyran-2-yl] (2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
1. Dura Life Saffron
2. Saffron Oil
3. Organic Saffron
4. Saffron
| Molecular Weight | 977.0 g/mol |
|---|---|
| Molecular Formula | C44H64O24 |
| XLogP3 | -2.5 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 20 |
| Exact Mass | 976.37875290 g/mol |
| Monoisotopic Mass | 976.37875290 g/mol |
| Topological Polar Surface Area | 391 Ų |
| Heavy Atom Count | 68 |
| Formal Charge | 0 |
| Complexity | 1730 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 20 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 7 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Crocin is a carotenoid constituent of saffron has also shown various pharmacological activities such as antioxidant, anticancer, memory improvement, antidepressant, cerebral, kidney, heart, skeletal muscle anti-ischemia, hypotensive , aphrodisiac, genoprotective and antidote activities. Crocin also inhibit morphine withdrawal syndrome and morphine-induced reinstatement of place preference in mice.
MohamadpourAH et al; Iran J Basic Med Sci. 16(1): 39-46 (2013)
EXPL THER Snakebite is a serious medical and socio-economic problem affecting the healthy individuals and agricultural and farming populations worldwide. In India, Vipera russelli snakebite is common, ensuing high morbidity and mortality. The venom components persuade multifactorial stress phenomenon and alter the physiological setting by causing disruption of the blood cells and vital organs. The present study demonstrates the anti-ophidian property of Crocin (Crocus sativus), a potent antioxidant against viper venom-induced oxidative stress. The in vivo oxidative damage induced by venom was clearly evidenced by the increased oxidative stress markers and antioxidant enzymes/molecules along with the proinflammatory cytokines including IL-1beta, TNF-a and IL-6. Furthermore, venom depleted the hemoglobin, hematocrit, mean corpuscular volume and platelet count in experimental animals. Crocin ameliorated the venom-induced oxidative stress, hematological alteration and proinflammatory cytokine levels. At present, administration of antivenom is an effective therapy against systemic toxicity, but it offers no protection against the rapidly spreading oxidative damage and infiltration of pro-inflammatory mediators. These pathologies will continue even after antivenom administration. Hence, a long-term auxiliary therapy is required to treat secondary as well as neglected complications of snakebite.
PMID:22893269 Santhosh SM et al; Cell Biochem Funct. 31 (1): 41-50 (2013)
EXPL THER The snakebite mortality rate has been significantly reduced due to effective antivenom therapy. The intravenously infused antivenom will neutralize free and target-bound toxins but fails to neutralize venom-induced inflammation and oxidative stress, as the antigen-antibody complex itself is pro-inflammatory. Therefore, an auxiliary therapy is necessary to treat secondary/overlooked envenomation complications. Blood samples from healthy donors were treated with viper venom (100 ug/mL) for 2 hr. The venom-induced inflammation, oxidative damage and effect of crocin pre-treatment were determined by assessing the serum levels of cytoplasmic, lysosomal and oxidative stress markers along with pro-inflammatory mediators such as tumor necrosis factor (TNF)-a, interleukin (IL)-1beta, IL-6 and cyclo-oxygenase (COX)-2. Significantly increased stress markers, cytoplasmic, lysosomal and extracellular matrix-degrading enzymes as well as the pro-inflammatory mediators TNF-a, IL-1beta, IL-6 and COX-2 indicated increased cellular damage but significantly reduced oxidative damage and inflammation in crocin pre-treated groups. CONCLUSION: The data clearly suggest that venom-induced oxidative stress and inflammation is also responsible for oxidative burst and cell death in the circulation, which may worsen even after antivenom therapy. Hence, the current study demands a supportive therapy in addition to antivenom therapy to neutralize the overlooked issues of snakebite.
PMID:23657249 Santhosh MS et al; Inflamm Res. 62 (7): 721-31 (2013)
EXPL THER /The study/ used an experimental model in the rat to examine the effects of long-term treatment with crocin, a glycosylated carotenoid from the stigmas of the saffron crocus, on colon cancer. BD-IX rats were divided into four groups: Groups G1 and G2, designated "cancer groups," were used to study the effects of crocin on the progression of colon cancer, and Groups G3 and G4, designated "toxicity groups," were used to study the effects of the treatment on metabolic processes and the parenchyma. DHD/K12-PROb cells were injected subcutaneously into the chest of Group G1 and G2 animals. From 1 to 13 weeks after inoculation, animals in Groups G2 and G4 received a weekly injection of crocin (400 mg/kg body wt s.c.). Animals in Groups G1 and G3 received no treatment. In addition, lines of animal and human colon adenocarcinoma cells (DHD/K12-PROb and HT-29) were used to perform assays in vitro to examine the cytotoxicity of crocin. Life span was extended and tumor growth was slower in crocin-treated female rats, but no significant antitumor effect was found in male rats. Acute tubular necrosis was found in all kidney samples from crocin-treated animals, but slight signs of nephrotoxicity were found by biochemical analysis of the serum. In assays in vitro, crocin had a potent cytotoxic effect on human and animal adenocarcinoma cells (HT-29 and DHD/K12-PROb cells, 50% lethal dose = 0.4 and 1.0 mM, respectively). Treated cells exhibited a remarkable loss of cytoplasm and wide cytoplasmic vacuole-like areas. In conclusion, long-term treatment with crocin enhances survival selectively in female rats with colon cancer without major toxic effects. The effects of crocin might be related to its strong cytotoxic effect on cultured tumor cells.
PMID:10693164 Garcia-Olmo DC et al; Nutr Cancer. 35 (2): 120-6 (1999)
EXPL THER Crocus sativus L. (saffron) has been traditionally used for the treatment of insomnia and other diseases of the nervous systems. Two carotenoid pigments, crocin and crocetin, are the major components responsible for the various pharmacological activities of C. sativus L. This study examined the sleep-promoting activity of crocin and crocetin by monitoring the locomotor activity and electroencephalogram after administration of these components to mice. Crocin (30 and 100 mg/kg) increased the total time of non-rapid eye movement (non-REM) sleep by 60 and 170%, respectively, during a 4-hr period from 20:00 to 24:00 after its intraperitoneal administration at a lights-off time of 20:00. Crocetin (100 mg/kg) also increased the total time of non-REM sleep by 50% after the administration. These compounds did not change the amount of REM sleep or show any adverse effects, such as rebound insomnia, after the induction of sleep.
PMID:22038919 Masaki M et al; Mol Nutr Food Res. 56 (2): 304-8 (2012)
This study investigated the pharmacokinetic properties of crocin following oral administration in rats. After a single oral dose, crocin was undetected while crocetin, a metabolite of crocin, was found in plasma at low concentrations. Simultaneously, crocin was largely present in feces and intestinal contents within 24 hr. After repeated oral doses for 6 days, crocin remained undetected in plasma and plasma crocetin concentrations were comparable to the corresponding data obtained after the single oral dose. Furthermore, the absorption characteristics of crocin were evaluated in situ using an intestinal recirculation perfusion method. During recirculation, crocin was undetected and low concentrations of crocetin were detected in plasma. The concentrations of crocin in the perfusate were reduced through different intestinal segments, and the quantities of drug lost were greater throughout the colon. These results indicate that (1) orally administered crocin is not absorbed either after a single dose or repeated doses, (2) crocin is excreted largely through the intestinal tract following oral administration, (3) plasma crocetin concentrations do not tend to accumulate with repeated oral doses of crocin, and (4) the intestinal tract serves as an important site for crocin hydrolysis.
PMID:17215113 XI L et al; Phytomedicine 14 (9): 633-6 (2007)
This study investigated the pharmacokinetic properties of crocin following oral administration in rats. After a single oral dose, crocin was undetected while crocetin, a metabolite of crocin, was found in plasma at low concentrations. Simultaneously, crocin was largely present in feces and intestinal contents within 24 hr.
PMID:17215113 XI L et al; Phytomedicine 14 (9): 633-6 (2007)
Crocin, the main pigment of Crocus sativus L., has been shown to have antiproliferative effects on cancer cells, but the involved mechanisms are only poor understood. This study focused on probable effect of crocin on the immortality of hepatic cancer cells. Cytotoxicity of crocin (IC50 3 mg/mL) in hepatocarcinoma HepG2 cells was determined after 48 hr by neutral red uptake assay and MTT test. Immortality was investigated through quantification of relative telomerase activity with a quantitative real-time PCR-based telomerase repeat amplification protocol (qTRAP). Telomerase activity in 0.5 ug protein extract of HepG2 cells treated with 3 mg/mL crocin was reduced to about 51% as compared to untreated control cells. Two mechanisms of inhibition, i.e. interaction of crocin with telomeric quadruplex sequences and down regulation of hTERT expression, were examined using FRET analysis to measure melting temperature of a synthetic telomeric oligonucleotide in the presence of crocin and quantitative real-time RT-PCR, respectively. No significant changes were observed in the Tm telomeric oligonucleotides, while the relative expression level of the catalytic subunit of telomerase (hTERT) gene showed a 60% decrease as compared to untreated control cells. In conclusion, telomerase activity of HepG2 cells decreases after treatment with crocin, which is probably caused by down-regulation of the expression of the catalytic subunit of the enzyme.
PMID:22901211 Noureini SK, Wink M.; Asian Pac J Cancer Prev. 13 (5): 2305-9 (2012)
Background Traditional drug discovery approaches are mainly relied on the observed phenotypic changes following administration of a plant extract, drug candidate or natural product. Recently, target-based approaches are becoming more popular. The present study aimed to identify the cellular targets of crocin, the bioactive dietary carotenoid present in saffron, using an affinity-based method. Methods Heart, kidney and brain tissues of BALB/c mice were homogenized and extracted for the experiments. Target deconvolution was carried out by first passing cell lysate through an affinity column prepared by covalently attaching crocin to agarose beads. Isolated proteins were separated on a 2D gel, trypsinized in situ and identified by MALDI-TOF/TOF mass spectrometry. MASCOT search engine was used to analyze Mass Data. Results Part of proteome that physically interacts with crocin was found to consist of beta-actin-like protein 2, cytochrome b-c1 complex subunit 1, ATP synthase subunit beta, tubulin beta-3 chain, tubulin beta-6 chain, 14-3-3 protein beta/alpha, V-type proton ATPase catalytic subunitA, 60 kDa heat shock protein, creatine kinase b-type, peroxiredoxin-2, cytochrome b-c1 complex subunit 2, acetyl-coA acetyltransferase, cytochrome c1, proteasome subunit alpha type-6 and proteasome subunit alpha type-4. Conclusion The present findings revealed that crocin physically binds to a wide range of cellular proteins such as structural proteins, membrane transporters, and enzymes involved in ATP and redox homeostasis and signal transduction.
Hosseinzadeh H et al; Daru. 22 (1): 5 (2014)
...treatment of PC-12 cells with crocin inhibited cell membrane lipid peroxidation and restored intracellular /Superoxide dismutase/ SOD activity even more efficacious than a-tocopherol at the same concentration. Further, in vitro studies demonstrated that the underlying mechanism through which crocin combats ischemic stress-induced neural cell death is by increasing /glutathione peroxidase/ GSH activities and preventing the activation of c-Jun NH2-terminal kinases (JNK) pathway...
Alavizadeh SH, Hosseinzadeh H; Food and Chemical Toxicology 64: 65-80 (2014) https://www.clearsightclearmind.com/files/Alavizadeh_2014.pdf


