
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
0
Australia
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
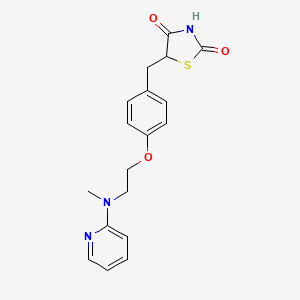

1. 5-((4-(2-methyl-2-(pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione-2-butenedioate
2. Avandia
3. Brl 49653
4. Brl-49653
5. Brl49653
6. Rosiglitazone Maleate
1. 122320-73-4
2. Avandia
3. Rosiglizole
4. 5-(4-(2-(methyl(pyridin-2-yl)amino)ethoxy)benzyl)thiazolidine-2,4-dione
5. Brl-49653
6. Brl 49653
7. Rezult
8. Brl49653
9. Rosiglitazone (avandia)
10. Avandaryl
11. Tdz 01
12. Gaudil
13. Rosvel
14. Avandamet
15. C18h19n3o3s
16. Chebi:50122
17. 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione
18. Mfcd00871760
19. Nsc-758698
20. Rosigilitazone
21. 2,4-thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-
22. 5-((4-(2-methyl-2-(pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione-2-butenedioate
23. 05v02f2kdg
24. Tdz-01
25. Rosiglitazone (inn)
26. Ncgc00095124-01
27. Brl 49653c
28. 5-(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}benzyl)-1,3-thiazolidine-2,4-dione
29. Rosiglitazone [inn]
30. 2,4-thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-
31. 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
32. Dsstox_cid_17131
33. Dsstox_rid_79303
34. Dsstox_gsid_37131
35. 5-[4-[2-[methyl(2-pyridyl)amino]ethoxy]benzyl]thiazolidine-2,4-dione
36. Rosiglitazone [inn:ban]
37. 5-[4-[2-(n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione
38. 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione
39. 5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione
40. Cas-122320-73-4
41. Sr-01000763023
42. Rosiglitazona
43. Rosiglitazonum
44. Unii-05v02f2kdg
45. Rosi
46. Dtxsid7037131
47. Hsdb 7555
48. Rosiglitazone Base
49. 5-[4-[2-[n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione
50. Rgz
51. Gaudil (tn)
52. Rosiglitazone- Bio-x
53. Spectrum_001703
54. 1217260-35-9
55. Spectrum2_001241
56. Spectrum3_000997
57. Spectrum4_001125
58. Spectrum5_001464
59. Rosiglitazone [mi]
60. Schembl5169
61. Dioxopromethazinehydrochloride
62. Rosiglitazone [hsdb]
63. Rosiglitazone [iarc]
64. Bspbio_002693
65. Kbiogr_001609
66. Kbioss_002183
67. Rosiglitazone [vandf]
68. Rosiglitazone [mart.]
69. Spectrum1504263
70. Spbio_001142
71. Rosiglitazone [who-dd]
72. Gtpl1056
73. Rosiglitazone [ema Epar]
74. Schembl14383595
75. Kbio2_002183
76. Kbio2_004751
77. Kbio2_007319
78. Kbio3_001913
79. Rosiglitazone, >=98% (hplc)
80. Hms1922j11
81. Hms2094o13
82. Hms3649g08
83. Hms3656k16
84. Hms3744m11
85. Hms3871l03
86. Hms3884n08
87. Pharmakon1600-01504263
88. Act04332
89. Bcp03047
90. Tox21_111434
91. Bdbm50030474
92. Ccg-39102
93. Nsc758698
94. Stl350047
95. Akos015894872
96. Tox21_111434_1
97. Ac-3459
98. Bcp9000017
99. Cs-1088
100. Db00412
101. Nsc 758698
102. Sb17326
103. Ncgc00095124-02
104. Ncgc00095124-03
105. Ncgc00095124-04
106. Ncgc00095124-05
107. Ncgc00095124-06
108. Ncgc00095124-08
109. Br164372
110. Hy-17386
111. Sy031184
112. Bcp0726000232
113. Ft-0602578
114. R0106
115. S2556
116. Sw197573-6
117. 20r734
118. 6p-065
119. D08491
120. S00306
121. Ab00698473-15
122. Ab00698473-17
123. Ab00698473-18
124. Ab00698473-19
125. Ab00698473_20
126. Ab00698473_21
127. Ab00698473_22
128. Ab00698473_23
129. Q424771
130. Q-201681
131. Sr-01000763023-5
132. Sr-01000763023-6
133. Brd-a97437073-001-02-3
134. Brd-a97437073-001-03-1
135. Brd-a97437073-001-04-9
136. Sr-01000763023-12
137. (rs)-5-{4-[2-(methyl-2-pyridylamino)ethoxy]benzyl}-2,4-thiazolidinedion
138. 5-(4-(2-(methyl(pyridin-2-yl)amino)ethoxy)-benzyl)thiazolidine-2,4-dione
139. 5-[4-[2-(n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4dione
140. Idmb (1um Brl49653, 1um Dexamethasone, 0.5um Ibmx, 10ug/ml Insulin)
141. (+/-)-5-[p-[2-(methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione
142. 2,4-thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]- (9ci)
143. 5-(4-(2-(n-methyl-n-(2-pyridinyl)amino)ethoxy)benzyl)-2,4-thiazolidinedione
144. 5-[[4-[2-(methyl-(2-pyridyl)amino)ethoxy]phenyl]methyl] Thiazolidine-2,4-dione
145. 5-[[4-[2-(methyl-2-pyridinylamino) Ethoxy]phenyl]methyl]-2,4-thiazolidinedione
146. 5-[[4-[2-(methyl-2-pyridinylamino)e Thoxy]phenyl]methyl]-2,4-thiazolidinedione
147. 5-[[4-[2-(methyl-pyridin-2-ylamino)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
148. 5-[4-[2-(n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl] Thiazolidine-2,4-dione
149. 5-[4-[2-[n-methyl-n-(2-pyridyl)amino]ethoxy]phenyl Methyl]thiazolidine-2,4-dione
| Molecular Weight | 357.4 g/mol |
|---|---|
| Molecular Formula | C18H19N3O3S |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 357.11471265 g/mol |
| Monoisotopic Mass | 357.11471265 g/mol |
| Topological Polar Surface Area | 96.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 469 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Avandaryl |
| PubMed Health | Rosiglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | rosiglitazone maleate; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 2 of 6 | |
|---|---|
| Drug Name | Avandia |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 3 of 6 | |
|---|---|
| Drug Name | Rosiglitazone |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 8mg; 4mg; 2mg |
| Market Status | Tentative Approval |
| Company | Watson Labs; Dr Reddys Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Avandaryl |
| PubMed Health | Rosiglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | rosiglitazone maleate; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 5 of 6 | |
|---|---|
| Drug Name | Avandia |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 6 of 6 | |
|---|---|
| Drug Name | Rosiglitazone |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 8mg; 4mg; 2mg |
| Market Status | Tentative Approval |
| Company | Watson Labs; Dr Reddys Labs |
Antidiabetic agent
National Library of Medicine's Medical Subject Headings. Rosiglitazone. Online file (MeSH, 2018). Available from, as of January 22, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Rosiglitazone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of January 22, 2018: https://clinicaltrials.gov/
Rosiglitazone is used as monotherapy or in combination with a sulfonylurea, metformin hydrochloride, or a sulfonylurea and metformin as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus. Rosiglitazone in fixed combination with metformin hydrochloride is used as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus. Rosiglitazone also is used in fixed combination with glimepiride as an adjunct to diet and exercise for the management of type 2 diabetes mellitus. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3301
/EXPL THER/ 1-Methyl-4-phenylpyridinium ion (MPP(+)), an inhibitor of mitochondrial complex I, has been widely used as a neurotoxin because it elicits a severe Parkinson's disease-like syndrome with elevation of intracellular reactive oxygen species (ROS) level and apoptotic death. Rosiglitazone, a peroxisome proliferator-activated receptor (PPAR)-gamma agonist, has been known to show various non-hypoglycemic effects, including anti-inflammatory, anti-atherogenic, and anti-apoptotic. In the present study, /the authors/ investigated the protective effects of rosiglitazone on MPP(+) induced cytotoxicity in human neuroblastoma SH-SY5Y cells, as well as underlying mechanism. /Their/ results suggested that the protective effects of rosiglitazone on MPP(+) induced apoptosis may be ascribed to its anti-oxidative properties, anti-apoptotic activity via inducing expression of SOD and catalase and regulating the expression of Bcl-2 and Bax. These data indicated that rosiglitazone might provide a valuable therapeutic strategy for the treatment of progressive neurodegenerative disease such as Parkinson's disease.
PMID:17266988 Jung T, Lee J et al; J Neurol Sci 253 (1-2): 53-60 (2007)
/BOXED WARNING/ WARNING: CONGESTIVE HEART FAILURE. Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients. After initiation of Avandia, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain; dyspnea; and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of Avandia must be considered. Avandia is not recommended in patients with symptomatic heart failure. Initiation of Avandia in patients with established NYHA Class III or IV heart failure is contraindicated.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
Thiazolidinediones, including rosiglitazone, alone or in combination with other antidiabetic agents, can cause fluid retention and may lead to or exacerbate congestive heart failure (CHF). Use of thiazolidinediones is associated with an approximately twofold increased risk of CHF. Patients should be observed for signs and symptoms of CHF (e.g., dyspnea, rapid weight gain, edema, unexplained cough or fatigue), especially during initiation of therapy and dosage titration. If signs and symptoms of CHF develop, the disorder should be managed according to current standards of care. In addition, a decrease in the dosage of rosiglitazone or discontinuance of the drug should be considered.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3303
Thiazolidinedione use is associated with bone loss and fractures in women and possibly in men with type 2 diabetes mellitus. In long-term comparative clinical trials in patients with type 2 diabetes mellitus, the incidence of bone fracture was increased in patients (particularly women) receiving rosiglitazone versus comparator agents (glyburide and/or metformin). Such effects were noted after the first year of treatment and persisted throughout the study. The majority of fractures observed in patients taking thiazolidinediones were in a distal upper limb (i.e., forearm, hand, wrist) or distal lower limb (i.e., foot, ankle, fibula, tibia). In an observational study in the United Kingdom in men and women (mean age: 60.7 years) with diabetes mellitus, use of pioglitazone or rosiglitazone for approximately 12-18 months (as estimated from prescription records) was associated with a twofold to threefold increase in fractures, particularly of the hip and wrist. The overall risk of fracture was similar among men and women and was independent of body mass index, comorbid conditions, diabetic complications, duration of diabetes mellitus, and use of other oral antidiabetic drugs.145 Risk of fractures should be considered when initiating or continuing thiazolidinedione therapy in female patients with type 2 diabetes mellitus. Bone health should be assessed and maintained according to current standards of care. Although increased risk of fracture may also apply to men, the risk appears to be higher among women than men.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3304
Because rosiglitazone requires endogenous insulin for activity, it should not be used in patients with type 1 diabetes mellitus or diabetic ketoacidosis.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3302
For more Drug Warnings (Complete) data for Rosiglitazone (19 total), please visit the HSDB record page.
Rosiglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
FDA Label
AVANDAMET is indicated in the treatment of type 2 diabetes mellitus patients, particularly overweight patients:
- who are unable to achieve sufficient glycaemic control at their maximally tolerated dose of oral metformin alone.
- in triple oral therapy with sulphonylurea in patients with insufficient glycaemic control despite dual oral therapy with their maximally tolerated dose of metformin and a sulphonylurea (see section 4. 4).
Rosiglitazone is indicated in the treatment of type 2 diabetes mellitus:
as monotherapy
-in patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance
as dual oral therapy in combination with
-metformin, in patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin
-a sulphonylurea, only in patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite monotherapy with a sulphonylurea
as triple oral therapy in combination with
-metformin and a sulphonylurea, in patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy (see section 4. 4).
Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea:
- in combination with metformin particularly in overweight patients.
- in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated.
Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea:
- in combination with metformin particularly in overweight patients.
- in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated.
When rosiglitazone is used as monotherapy, it is associated with increases in total cholesterol, LDL, and HDL. It is also associated with decreases in free fatty acids. Increases in LDL occurred primarily during the first 1 to 2 months of therapy with AVANDIA and LDL levels remained elevated above baseline throughout the trials. In contrast, HDL continued to rise over time. As a result, the LDL/HDL ratio peaked after 2 months of therapy and then appeared to decrease over time.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BD03
A10BG02
A10BG02
A10BG02
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BG - Thiazolidinediones
A10BG02 - Rosiglitazone
Absorption
The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Administration of rosiglitazone with food resulted in no change in overall exposure (AUC), but there was an approximately 28% decrease in Cmax and a delay in Tmax (1.75 hours). These changes are not likely to be clinically significant; therefore, rosiglitazone may be administered with or without food. Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range.
Route of Elimination
Following oral or intravenous administration of [14C]rosiglitazone maleate, approximately 64% and 23% of the dose was eliminated in the urine and in the feces, respectively.
Volume of Distribution
17.6 L [oral volume of distribution Vss/F]
13.5 L [population mean, pediatric patients]
Clearance
Oral clearance (CL) = 3.03 0.87 L/hr [1 mg Fasting]
Oral CL = 2.89 0.71 L/hr [2 mg Fasting]
Oral CL = 2.85 0.69 L/hr [8 mg Fasting]
Oral CL = 2.97 0.81 L/hr [8 mg Fed]
3.15 L/hr [Population mean, Pediatric patients]
In a study in healthy volunteers, the absorption of rosiglitazone was relatively rapid, with 99% oral bioavailability after oral absorption.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 362 (2016)
Severe forms of non-alcoholic fatty liver disease (NAFLD) adversely affect the liver physiology and hence the pharmacokinetics of drugs. Here, we investigated the effect of NAFLD on the pharmacokinetics of rosiglitazone, an insulin sensitizer used in the treatment of type 2 diabetes. Male C57BL/6 mice were divided into two groups. The first group (n=14) was fed with normal chow feed and the second group (n=14) was fed with 60% high-fat diet (HFD) and 40% high fructose liquid (HFL) for 60 days to induce NAFLD. The development of NAFLD was confirmed by histopathology, liver triglyceride levels and biochemical estimations, and used for pharmacokinetic investigations. Rosiglitazone was administered orally at 30 mg/kg dose. At predetermined time points, blood was collected and rosiglitazone concentrations were determined using LC/MS/MS. Plasma concentrations were subjected to non-compartmental analysis using Phoenix WinNonlin (6.3), and the area under the plasma concentration-time curve (AUC) was calculated by the linear-up log-down method. HFD and HFL diet successfully induced NAFLD in mice. Rosiglitazone pharmacokinetics in NAFLD animals were altered significantly as compared to healthy mice. Rosiglitazone exposure increased significantly in NAFLD mice (2.5-fold higher AUC than healthy mice). The rosiglitazone oral clearance was significantly lower and the mean plasma half-life was significantly longer in NAFLD mice as compared to healthy mice. The NAFLD mouse model showed profound effects on rosiglitazone pharmacokinetics. The magnitude of change in rosiglitazone pharmacokinetics is similar to that observed in humans with moderate to severe liver disease. The present animal model can be utilized to study the NAFLD-induced changes in the pharmacokinetics of different drugs.
PMID:27522101 Kulkarni NM et al; Drug Metab Pers Ther 31 (3): 165-71 (2016)
The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Administration of rosiglitazone with food resulted in no change in overall exposure (AUC), but there was an approximately 28% decrease in Cmax and a delay in Tmax (1.75 hours). These changes are not likely to be clinically significant; therefore, Avandia may be administered with or without food.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
The mean (CV%) oral volume of distribution (Vss/F) of rosiglitazone is approximately 17.6 (30%) liters, based on a population pharmacokinetic analysis. Rosiglitazone is approximately 99.8% bound to plasma proteins, primarily albumin.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
For more Absorption, Distribution and Excretion (Complete) data for Rosiglitazone (8 total), please visit the HSDB record page.
Hepatic. Rosiglitazone is extensively metabolized in the liver to inactive metabolites via N-demethylation, hydroxylation, and conjugation with sulfate and glucuronic acid. In vitro data have shown that Cytochrome (CYP) P450 isoenzyme 2C8 (CYP2C8) and to a minor extent CYP2C9 are involved in the hepatic metabolism of rosiglitazone.
The main metabolites observed in humans are also observed in rats; however, the clearance in rats was almost ten times higher than in humans, probably due to the higher levels of CYP2C in rat microsomes.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 364 (2016)
In vitro data demonstrate that rosiglitazone is predominantly metabolized by Cytochrome P450 (CYP) isoenzyme 2C8, with CYP2C9 contributing as a minor pathway.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine. The major routes of metabolism were N-demethylation and hydroxylation, followed by conjugation with sulfate and glucuronic acid. All the circulating metabolites are considerably less potent than parent and, therefore, are not expected to contribute to the insulin-sensitizing activity of rosiglitazone.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
Rosiglitazone has known human metabolites that include N-Desmethylrosiglitazone, ortho-hydroxyrosiglitazone, and para-hydroxyrosiglitazone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
3-4 hours (single oral dose, independent of dose)
The elimination half-life of rosiglitazone was 3-4 hours and was independent of dose. The time to Cmax and the elimination half-life for two metabolites in plasma were significantly longer than for rosiglitazone itself (4-6 hours versus 0.5-1 hours, and about 5 days versus 3-7 hours).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 362 (2016)
The plasma half life of (14)C-related material ranged from 103 to 158 hours.
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
Rosiglitazone acts as a highly selective and potent agonist at peroxisome proliferator activated receptors (PPAR) in target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPAR-gamma receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In this way, rosiglitazone enhances tissue sensitivity to insulin.
Because osteoblasts and marrow adipocytes are derived from a common mesenchymal progenitor, increased adipogenesis may occur at the expense of osteoblasts, leading to bone loss. Our previous in vitro studies indicated that activation of the proadipogenic transcription factor peroxisome proliferator-activated receptor isoform gamma 2 with rosiglitazone suppressed osteoblast differentiation. Here, we show that 5-month-old Swiss-Webster mice receiving rosiglitazone for 28 day exhibited bone loss associated with an increase in marrow adipocytes, a decrease in the ratio of osteoblasts to osteoclasts, a reduction in bone formation rate, and a reduction in wall width--an index of the amount of bone formed by each team of osteoblasts. Rosiglitazone had no effect on the number of early osteoblast or osteoclast progenitors, or on osteoblast life span, but decreased the expression of the key osteoblastogenic transcription factors Runx2 and Osterix in cultures of marrow-derived mesenchymal progenitors. These effects were associated with diversion of bipotential progenitors from the osteoblast to the adipocyte lineage, and suppression of the differentiation of monopotential osteoblast progenitors. However, rosiglitazone had no effect on osteoblastic cells at later stages of differentiation. Hence, rosiglitazone attenuates osteoblast differentiation and thereby reduces bone formation rate in vivo, leading to bone loss. These findings provide a mechanistic explanation for the recent evidence that peroxisome proliferator-activated receptor isoform gamma activation is a negative regulator of bone mass and suggest that the increased production of oxidized fatty acids with age may indeed be an important mechanism for age-related osteoporosis in humans.
PMID:15591153 Ali AA et al; Endocrinology 146 (3): 1226-35 (2005)
Brain peroxisome proliferator-activated receptor gamma (PPARgamma), a member of the nuclear receptor superfamily of ligand-dependent transcription factors, is involved in neuroprotection. It is activated by the drug rosiglitazone, which then can increase the pro-survival protein B-cell lymphoma 2 (BCL-2), to mediate neuroprotection. However, the mechanism underlying this molecular cascade remains unknown. Here, we show that the neuroprotective protein neurotrophic factor-a1 (NF-a1), which also induces the expression of BCL-2, has a promoter that contains PPARgamma-binding sites that are activated by rosiglitazone. Treatment of Neuro2a cells and primary hippocampal neurons with rosiglitazone increased endogenous NF-a1 expression and prevented H2 O2 -induced cytotoxicity. Concomitant with the increase in NF-a1, BCL-2 was also increased in these cells. When siRNA against NF-a1 was used, the induction of BCL-2 by rosiglitazone was prevented, and the neuroprotective effect of rosiglitazone was reduced. These results demonstrate that rosiglitazone-activated PPARgamma directly induces the transcription of NF-a1, contributing to neuroprotection in neurons. We proposed the following cascade for neuroprotection against oxidative stress by rosiglitazone: Rosiglitazone enters the neuron and binds to peroxisome proliferator-activated receptor gamma (PPARgamma) in the nucleus. The PPARgamma-rosiglitazone complex binds to the neurotrophic factor-a1 (NF-a1) promoter and activates the transcription of NF-a1 mRNA which is then translated to the protein. NF-a1 is the secreted, binds to a cognate receptor and activates the extracellular signal-regulated kinases (ERK) pathway. This in turn enhances the expression of the pro-survival protein, B-cell lymphoma 2 (BCL-2) and inhibition of caspase 3 (Csp-3) to mediate neuroprotection under oxidative stress. Akt, protein kinase B (PKB).
PMID:25940785 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4496294 Thouennon E et al; J Neurochem 134 (3): 463-70 (2015)
Rosiglitazone, a member of the thiazolidinedione class of antidiabetic agents, improves glycemic control by improving insulin sensitivity. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator-activated receptor-gamma (PPARgamma). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARgamma nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARgamma- responsive genes also participate in the regulation of fatty acid metabolism
GlaxoSmithKline (GSK); Prescribing Information for Avandia (Rosiglitazone Maleate) Tablets, (Revised September 2016). Available from, as of January 30, 2018: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands.html?type=Prescription
Rosiglitazone acts principally by increasing insulin sensitivity in target tissues, as well as decreasing hepatic gluconeogenesis. Rosiglitazone is a peroxisome proliferator-activated receptorgamma (PPARgamma) agonist that increases transcription of insulin-responsive genes and increases insulin sensitivity. Rosiglitazone, like other thiazolidinediones, ameliorates insulin resistance associated with type diabetes mellitus without stimulating insulin release from pancreatic beta cells, thus avoiding the risk of hypoglycemia. Because rosiglitazone does not lower glucose concentrations below euglycemia, the drug is appropriately referred to as an antidiabetic agent rather than a hypoglycemic agent. Some evidence suggests that the glucoregulatory effects of thiazolidinediones are mediated in part via reduced systemic and tissue lipid availability. Circulating concentrations of insulin and C-peptide are reduced during rosiglitazone therapy.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3305

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
48
PharmaCompass offers a list of Rosiglitazone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rosiglitazone manufacturer or Rosiglitazone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rosiglitazone manufacturer or Rosiglitazone supplier.
PharmaCompass also assists you with knowing the Rosiglitazone API Price utilized in the formulation of products. Rosiglitazone API Price is not always fixed or binding as the Rosiglitazone Price is obtained through a variety of data sources. The Rosiglitazone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rosiglitazone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rosiglitazone, including repackagers and relabelers. The FDA regulates Rosiglitazone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rosiglitazone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rosiglitazone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rosiglitazone supplier is an individual or a company that provides Rosiglitazone active pharmaceutical ingredient (API) or Rosiglitazone finished formulations upon request. The Rosiglitazone suppliers may include Rosiglitazone API manufacturers, exporters, distributors and traders.
click here to find a list of Rosiglitazone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rosiglitazone DMF (Drug Master File) is a document detailing the whole manufacturing process of Rosiglitazone active pharmaceutical ingredient (API) in detail. Different forms of Rosiglitazone DMFs exist exist since differing nations have different regulations, such as Rosiglitazone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rosiglitazone DMF submitted to regulatory agencies in the US is known as a USDMF. Rosiglitazone USDMF includes data on Rosiglitazone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rosiglitazone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rosiglitazone suppliers with USDMF on PharmaCompass.
Rosiglitazone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rosiglitazone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rosiglitazone GMP manufacturer or Rosiglitazone GMP API supplier for your needs.
A Rosiglitazone CoA (Certificate of Analysis) is a formal document that attests to Rosiglitazone's compliance with Rosiglitazone specifications and serves as a tool for batch-level quality control.
Rosiglitazone CoA mostly includes findings from lab analyses of a specific batch. For each Rosiglitazone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rosiglitazone may be tested according to a variety of international standards, such as European Pharmacopoeia (Rosiglitazone EP), Rosiglitazone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rosiglitazone USP).

