



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
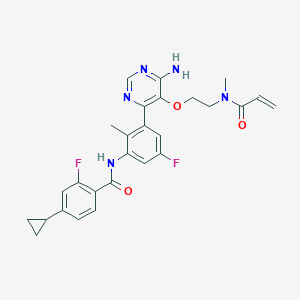

1. Lou064
2. N-(3-(6-amino-5-(2-(methyl(prop-2-enoyl)amino)ethoxy)pyrimidin-4-yl)-5-fluoro-2-methylphenyl)-4-cyclopropyl-2-fluorobenzamide
1. 1787294-07-8
2. Remibrutinib [inn]
3. Lou064
4. Remibrutinib [usan]
5. I7mvz8hdnu
6. Remibrutinib (lou064)
7. Nvp-lou064-nxa
8. Lou064-nxa
9. N-(3-(6-amino-5-(2-(n-methylacrylamido)ethoxy)pyrimidin-4-yl)-5-fluoro-2-methylphenyl)-4-cyclopropyl-2-fluorobenzamide
10. N-[3-[6-amino-5-[2-[methyl(1-oxo-2-propen-1-yl)amino]ethoxy]-4-pyrimidinyl]-5-fluoro-2-methylphenyl]-4-cyclopropyl-2-fluorobenzamide
11. N-[3-[6-amino-5-[2-[methyl(prop-2-enoyl)amino]ethoxy]pyrimidin-4-yl]-5-fluoro-2-methylphenyl]-4-cyclopropyl-2-fluorobenzamide
12. Benzamide, N-(3-(6-amino-5-(2-(methyl(1-oxo-2-propen-1-yl)amino)ethoxy)-4-pyrimidinyl)-5-fluoro-2-methylphenyl)-4-cyclopropyl-2-fluoro-
13. Unii-i7mvz8hdnu
14. Chembl4483575
15. Schembl16754888
16. Gtpl10457
17. Lou-064
18. Ex-a3421
19. S9660
20. Who 11062
21. Example 6 [wo2015079417a1]
22. Compound 25 [pmid: 32083858]
23. Ac-36985
24. Hy-128757
25. Cs-0103905
26. Us9512084, 6
27. A930622
28. Lou-064;n-(3-(6-amino-5-(2-(n-methylacrylamido)ethoxy)pyrimidin-4-yl)-5-fluoro-2-methylphenyl)-4-cyclopropyl-2-fluorobenzamide
| Molecular Weight | 507.5 g/mol |
|---|---|
| Molecular Formula | C27H27F2N5O3 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 507.20819606 g/mol |
| Monoisotopic Mass | 507.20819606 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 815 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)


