


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
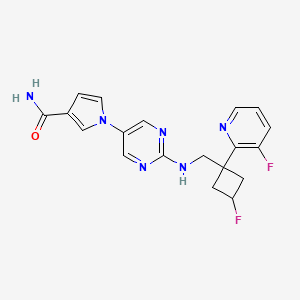

1. 1-(2-((3-fluoro-1-(3-fluoropyridin-2-yl)cyclobutyl)methylamino)pyrimidin-5-yl)pyrrole-3-carboxamide
2. Ck-2127107
1. Ck-2127107
2. 1345410-31-2
3. Reldesemtiv [usan]
4. 4s0hbyw6qe
5. Reldesemtiv (usan)
6. 1-[2-({[trans-3-fluoro-1-(3-fluoropyridin-2-yl)cyclobutyl]methyl}amino)pyrimidin-5-yl]-1h-pyrrole-3- Carboxamide
7. 1-[2-[[3-fluoro-1-(3-fluoropyridin-2-yl)cyclobutyl]methylamino]pyrimidin-5-yl]pyrrole-3-carboxamide
8. 1h-pyrrole-3-carboxamide, 1-(2-(((trans-3-fluoro-1-(3-fluoro-2-pyridinyl)cyclobutyl)methyl)amino)-5-pyrimidinyl)-
9. Unii-4s0hbyw6qe
10. Reldesemtiv [inn]
11. Schembl2566240
12. Chembl4297600
13. Schembl12288022
14. Schembl17584804
15. Gtpl11801
16. Ck-107
17. Db15256
18. Ck2127107
19. Hy-109121
20. Cs-0078343
21. J3.610.548h
22. D11363
23. 1-(2-(((trans-3-fluoro-1-(3-fluoro-2-pyridinyl)cyclobutyl)methyl)amino)-5-pyrimidinyl)- 1h-pyrrole-3-carboxamide
24. 1-(2-(((trans-3-fluoro-1-(3-fluoropyridin-2-yl)cyclobutyl)methyl)amino)pyrimidin-5-yl)-1h-pyrrole-3-carboxamide
25. 1-[2-[[[3alpha-fluoro-1beta-(3-fluoro-2-pyridyl)cyclobutane-1-yl]methyl]amino]pyrimidine-5-yl]-1h-pyrrole-3-carboxamide
26. 1-[2-[[[trans-3-fluoro-1-(3-fluoro-2-pyridinyl)cyclobutyl]methyl]amino]-5-pyrimidinyl]-1h-pyrrole-3-carboxamide
| Molecular Weight | 384.4 g/mol |
|---|---|
| Molecular Formula | C19H18F2N6O |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 384.15101554 g/mol |
| Monoisotopic Mass | 384.15101554 g/mol |
| Topological Polar Surface Area | 98.7 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 553 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of amyotrophic lateral sclerosis


