



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
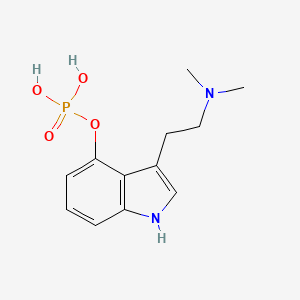

1. Psilocibin
2. Psilocybin
1. Psilocybin
2. Indocybin
3. Psilocibin
4. Teonanacatl
5. 520-52-5
6. Psilocin Phosphate Ester
7. Psilotsibin
8. Psilocibina
9. Psilocybinum
10. O-phosphoryl-4-hydroxy-n,n-dimethyltryptamine
11. Cy 39
12. Cy-39
13. 3-(2-dimethylaminoethyl)indol-4-yl Dihydrogen Phosphate
14. 4-phosphoryloxy-n,n-dimethyltryptamine
15. 3-2'-dimethylaminoethylindol-4-phosphate
16. 3-(2-(dimethylamino)ethyl)-1h-indol-4-ol Dihydrogen Phosphate Ester
17. Chebi:8614
18. Chembl194378
19. 3-[2-(dimethylamino)ethyl]-1h-indol-4-yl Dihydrogen Phosphate
20. 2rv7212bp0
21. 1h-indol-4-ol, 3-(2-(dimethylamino)ethyl)-, Dihydrogen Phosphate (ester)
22. Ncgc00247732-01
23. Indol-4-ol, 3-(2-(dimethylamino)ethyl)-, Dihydrogen Phosphate
24. Psylocybin
25. Psilocybinum [inn-latin]
26. Psilocibina [inn-spanish]
27. Hsdb 7365
28. Einecs 208-294-4
29. Brn 0273158
30. Psilocybine [inn:ban:dcf]
31. Unii-2rv7212bp0
32. [3-[2-(dimethylamino)ethyl]-1h-indol-4-yl] Dihydrogen Phosphate
33. 4-phosphoryloxy-omega-n,n-dimethyltryptamine
34. Dea No. 7437
35. (3-(2-(dimethylamino)ethyl)-1h-indol-4-yl) Dihydrogen Phosphate
36. 1h-indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate (ester)
37. Psilocybin [mi]
38. Psilocybine [inn]
39. Psilocybine [hsdb]
40. Dsstox_cid_28824
41. Dsstox_rid_83093
42. Psilocybine [mart.]
43. Dsstox_gsid_48898
44. Psilocybine [who-dd]
45. 4-22-00-05665 (beilstein Handbook Reference)
46. Constituent Of Magic Mushrooms
47. Schembl158945
48. Cy39
49. Dtxsid0048898
50. Zinc1530830
51. Tox21_112898
52. Bdbm50171269
53. Pdsp1_001391
54. Pdsp2_001375
55. Db11664
56. Sb18760
57. Cas-520-52-5
58. C07576
59. P-7825
60. Q208118
61. 3-[2-(dimethylamino)ethyl]indol-4-yl Dihydrogen Phosphate
62. ({3-[2-(dimethylamino)ethyl]-1h-indol-4-yl}oxy)phosphonic Acid
63. 1h-indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate
64. 3-[2-(dimethylamino)ethyl]-1h-indol-4-yl Dihydrogen Phosphate #
65. 3-[2-(dimethylamino)ethyl]indol-4-ol Dihydrogen Phosphate Ester
66. Indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate (ester)
| Molecular Weight | 284.25 g/mol |
|---|---|
| Molecular Formula | C12H17N2O4P |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 284.09259403 g/mol |
| Monoisotopic Mass | 284.09259403 g/mol |
| Topological Polar Surface Area | 85.8 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 347 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
In a clinical study eight volunteers received psilocybin in psychoactive oral doses of 212+ or -25 ug/kg body weight. To investigate the elimination kinetics of psilocin, the first metabolite of psilocybin, urine was collected for 24 hr and psilocin concentrations were determined by high-performance liquid chromatography with column switching and electrochemical detection (HPLC-ECD). Sample workup included protection of the unstable psilocin with ascorbic acid, freeze-drying, and extraction with methanol. Peak psilocin concentrations up to 870 ug/l were measured in urine samples from the 2-4 hr collection interval. The psilocin excretion rate in this period was 55.5+ or -33.8 microg/h. The limit of quantitation (10 ug/L) was usually reached 24 hr after drug administration. Within 24 hr, 3.4+ or -0.9% of the applied dose of psilocybin was excreted as free psilocin. Addition of beta-glucuronidase to urine samples and incubation for 5 hr at 40 degrees C led to twofold higher psilocin concentrations, although 18+ or -7% of the amount of unconjugated PI was decomposed during incubation. /In conclusion/ that in humans psilocin is partially excreted as psilocin-O-glucuronide and that enzymatic hydrolysis extends the time of detectability for psilocin in urine samples.
PMID:12191719 Hasler F et al; J Pharm Biomed Anal 30 (2): 331-9 (2002)
Psilocybin is rapidly and completely hydrolyzed to psilocin in vivo.
Goldfrank, L.R. (ed). Goldfrank's Toxicologic Emergencies. 7th Edition McGraw-Hill New York, New York 2002., p. 1121
Psilocybin, which resembles 5-hydroxytryptamine and LSD, inhibits the firing of serotonin-dependent neurons, causing alterations in perception, changes in mood, hallucinations, and a distortion of time.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 906
The active constituents of hallucinogenic mushrooms probably are indole compounds derived from tryptamine. Psilocybin and its less stable metabolite psilocin are the best known active compounds, but their effects may not entirely explain the range of symptoms caused by hallucinogenic mushrooms. Wide variations occurs in clinical response.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1733


