ACTIVE PHARMA INGREDIENTS
DEVELOPMENTS
DIGITAL CONTENT
MARKET PLACE

Synopsis
Synopsis
ACTIVE PHARMA INGREDIENTS
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0INTERMEDIATES
REF. STANDARDS OR IMPURITIES
0
EDQM
0
USP
0
JP
0
Others
FINISHED DOSAGE FORMULATIONS
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0RELATED EXCIPIENT COMPANIES
0EXCIPIENTS BY APPLICATIONS
PATENTS & EXCLUSIVITIES
0
US Patents
0
US Exclusivities
0
Health Canada Patents
API REF. PRICE (USD/KG)
$ 0
GLOBAL SALES INFORMATION
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
MARKET PLACE
0
FDF
DIGITAL CONTENT
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
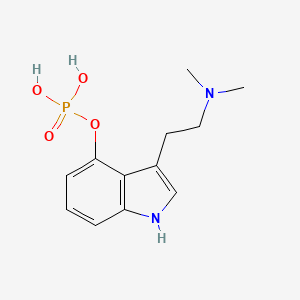

1. Psilocibin
2. Psilocybin
1. Psilocybin
2. Indocybin
3. Psilocibin
4. Teonanacatl
5. 520-52-5
6. Psilocin Phosphate Ester
7. Psilotsibin
8. Psilocibina
9. Psilocybinum
10. O-phosphoryl-4-hydroxy-n,n-dimethyltryptamine
11. Cy 39
12. Cy-39
13. 3-(2-dimethylaminoethyl)indol-4-yl Dihydrogen Phosphate
14. 4-phosphoryloxy-n,n-dimethyltryptamine
15. 3-2'-dimethylaminoethylindol-4-phosphate
16. 3-(2-(dimethylamino)ethyl)-1h-indol-4-ol Dihydrogen Phosphate Ester
17. Chebi:8614
18. Chembl194378
19. 3-[2-(dimethylamino)ethyl]-1h-indol-4-yl Dihydrogen Phosphate
20. 2rv7212bp0
21. 1h-indol-4-ol, 3-(2-(dimethylamino)ethyl)-, Dihydrogen Phosphate (ester)
22. Ncgc00247732-01
23. Indol-4-ol, 3-(2-(dimethylamino)ethyl)-, Dihydrogen Phosphate
24. Psylocybin
25. Psilocybinum [inn-latin]
26. Psilocibina [inn-spanish]
27. Hsdb 7365
28. Einecs 208-294-4
29. Brn 0273158
30. Psilocybine [inn:ban:dcf]
31. Unii-2rv7212bp0
32. [3-[2-(dimethylamino)ethyl]-1h-indol-4-yl] Dihydrogen Phosphate
33. 4-phosphoryloxy-omega-n,n-dimethyltryptamine
34. Dea No. 7437
35. (3-(2-(dimethylamino)ethyl)-1h-indol-4-yl) Dihydrogen Phosphate
36. 1h-indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate (ester)
37. Psilocybin [mi]
38. Psilocybine [inn]
39. Psilocybine [hsdb]
40. Dsstox_cid_28824
41. Dsstox_rid_83093
42. Psilocybine [mart.]
43. Dsstox_gsid_48898
44. Psilocybine [who-dd]
45. 4-22-00-05665 (beilstein Handbook Reference)
46. Constituent Of Magic Mushrooms
47. Schembl158945
48. Cy39
49. Dtxsid0048898
50. Zinc1530830
51. Tox21_112898
52. Bdbm50171269
53. Pdsp1_001391
54. Pdsp2_001375
55. Db11664
56. Sb18760
57. Cas-520-52-5
58. C07576
59. P-7825
60. Q208118
61. 3-[2-(dimethylamino)ethyl]indol-4-yl Dihydrogen Phosphate
62. ({3-[2-(dimethylamino)ethyl]-1h-indol-4-yl}oxy)phosphonic Acid
63. 1h-indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate
64. 3-[2-(dimethylamino)ethyl]-1h-indol-4-yl Dihydrogen Phosphate #
65. 3-[2-(dimethylamino)ethyl]indol-4-ol Dihydrogen Phosphate Ester
66. Indol-4-ol, 3-[2-(dimethylamino)ethyl]-, Dihydrogen Phosphate (ester)
| Molecular Weight | 284.25 g/mol |
|---|---|
| Molecular Formula | C12H17N2O4P |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 284.09259403 g/mol |
| Monoisotopic Mass | 284.09259403 g/mol |
| Topological Polar Surface Area | 85.8 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 347 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
In a clinical study eight volunteers received psilocybin in psychoactive oral doses of 212+ or -25 ug/kg body weight. To investigate the elimination kinetics of psilocin, the first metabolite of psilocybin, urine was collected for 24 hr and psilocin concentrations were determined by high-performance liquid chromatography with column switching and electrochemical detection (HPLC-ECD). Sample workup included protection of the unstable psilocin with ascorbic acid, freeze-drying, and extraction with methanol. Peak psilocin concentrations up to 870 ug/l were measured in urine samples from the 2-4 hr collection interval. The psilocin excretion rate in this period was 55.5+ or -33.8 microg/h. The limit of quantitation (10 ug/L) was usually reached 24 hr after drug administration. Within 24 hr, 3.4+ or -0.9% of the applied dose of psilocybin was excreted as free psilocin. Addition of beta-glucuronidase to urine samples and incubation for 5 hr at 40 degrees C led to twofold higher psilocin concentrations, although 18+ or -7% of the amount of unconjugated PI was decomposed during incubation. /In conclusion/ that in humans psilocin is partially excreted as psilocin-O-glucuronide and that enzymatic hydrolysis extends the time of detectability for psilocin in urine samples.
PMID:12191719 Hasler F et al; J Pharm Biomed Anal 30 (2): 331-9 (2002)
Psilocybin is rapidly and completely hydrolyzed to psilocin in vivo.
Goldfrank, L.R. (ed). Goldfrank's Toxicologic Emergencies. 7th Edition McGraw-Hill New York, New York 2002., p. 1121
Psilocybin, which resembles 5-hydroxytryptamine and LSD, inhibits the firing of serotonin-dependent neurons, causing alterations in perception, changes in mood, hallucinations, and a distortion of time.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 906
The active constituents of hallucinogenic mushrooms probably are indole compounds derived from tryptamine. Psilocybin and its less stable metabolite psilocin are the best known active compounds, but their effects may not entirely explain the range of symptoms caused by hallucinogenic mushrooms. Wide variations occurs in clinical response.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1733
Market Place

ABOUT THIS PAGE
Looking for 520-52-5 / Psilocybine API manufacturers, exporters & distributors?
Psilocybine manufacturers, exporters & distributors 123
PharmaCompass offers a list of Psilocybine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Psilocybine manufacturer or Psilocybine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Psilocybine manufacturer or Psilocybine supplier.
PharmaCompass also assists you with knowing the Psilocybine API Price utilized in the formulation of products. Psilocybine API Price is not always fixed or binding as the Psilocybine Price is obtained through a variety of data sources. The Psilocybine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
API | Excipient name
Synonyms
Cas Number
Unique Ingredient Identifier (UNII)
About Psilocybine
Psilocybine Manufacturers
A Psilocybine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Psilocybine, including repackagers and relabelers. The FDA regulates Psilocybine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Psilocybine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Psilocybine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
Psilocybine Suppliers
A Psilocybine supplier is an individual or a company that provides Psilocybine active pharmaceutical ingredient (API) or Psilocybine finished formulations upon request. The Psilocybine suppliers may include Psilocybine API manufacturers, exporters, distributors and traders.
click here to find a list of Psilocybine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Psilocybine USDMF
A Psilocybine DMF (Drug Master File) is a document detailing the whole manufacturing process of Psilocybine active pharmaceutical ingredient (API) in detail. Different forms of Psilocybine DMFs exist exist since differing nations have different regulations, such as Psilocybine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Psilocybine DMF submitted to regulatory agencies in the US is known as a USDMF. Psilocybine USDMF includes data on Psilocybine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Psilocybine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Psilocybine suppliers with USDMF on PharmaCompass.
Psilocybine GMP
Psilocybine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Psilocybine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Psilocybine GMP manufacturer or Psilocybine GMP API supplier for your needs.
Psilocybine CoA
A Psilocybine CoA (Certificate of Analysis) is a formal document that attests to Psilocybine's compliance with Psilocybine specifications and serves as a tool for batch-level quality control.
Psilocybine CoA mostly includes findings from lab analyses of a specific batch. For each Psilocybine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Psilocybine may be tested according to a variety of international standards, such as European Pharmacopoeia (Psilocybine EP), Psilocybine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Psilocybine USP).
For your convenience, we have listed synonyms and CAS numbers to help you find the best supplier. The use of synonyms and CAS numbers can be helpful in identifying potential suppliers, but it is crucial to note that they might not always indicate the exact same product. It is important to confirm the product details with the supplier before making a purchase to ensure that it meets your requirements.

What are you looking for?
- Privacy policy
- Terms and conditions
- Disclaimers
-

- Product listings are provided for informational purposes only. We do not supply or sell any products. Any products that may be covered by patent(s) are supplied solely for uses permitted under Section 107A of the Indian Patents Act and not for commercial sale.







