
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book
0
Australia
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
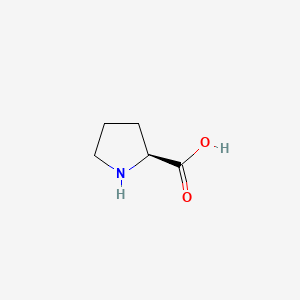

1. L Proline
2. L-proline
1. L-proline
2. 147-85-3
3. L-(-)-proline
4. (s)-pyrrolidine-2-carboxylic Acid
5. (2s)-pyrrolidine-2-carboxylic Acid
6. H-pro-oh
7. 2-pyrrolidinecarboxylic Acid
8. (-)-proline
9. (-)-(s)-proline
10. (s)-2-pyrrolidinecarboxylic Acid
11. Prolinum
12. (-)-2-pyrrolidinecarboxylic Acid
13. L-pyrrolidine-2-carboxylic Acid
14. Prolina
15. (s)-proline
16. L-alpha-pyrrolidinecarboxylic Acid
17. L-prolin
18. Prolinum [latin]
19. Prolina [spanish]
20. Proline, L-
21. Proline (van)
22. (s)-2-carboxypyrrolidine
23. Proline [usan:inn]
24. Fema No. 3319
25. (l)-proline
26. (s)-(-)-proline
27. 2-pyrrolidinecarboxylic Acid, (s)-
28. Fema Number 3319
29. Cb 1707
30. Pro (iupac Abbreviation)
31. L-proline, Labeled With Carbon-14
32. Hsdb 1210
33. Ai3-26710
34. 9dlq4ciu6v
35. Chembl54922
36. 37159-97-0
37. 4305-67-3
38. Chebi:17203
39. Nsc-46703
40. Proline (l-proline)
41. Mfcd00064318
42. Carboxypyrrolidine
43. L-(2,3-3h)proline
44. Proline (usp)
45. (2s)-pyrrolidin-1-ium-2-carboxylate
46. Einecs 205-702-2
47. Unii-9dlq4ciu6v
48. Nsc 46703
49. 2-pyrrolidinecarboxylate
50. Racemic Proline
51. Rac-proline
52. S-proline
53. 3h-l-proline
54. L-proline;
55. (s)-prolin
56. Femanumber3319
57. H-pro
58. (2s)-proline
59. Pro-oh
60. L-proline,(s)
61. L-pro-oh
62. (-)-proline (s)-2-carboxypyrrolidine
63. L-proline (jp17)
64. Proline [vandf]
65. Proline [hsdb]
66. Proline [inci]
67. Proline [usan]
68. Proline [inn]
69. Proline [ii]
70. Proline [mi]
71. L-proline [fcc]
72. L-proline [jan]
73. Proline [mart.]
74. L-proline [fhfi]
75. Proline [who-dd]
76. Bmse000047
77. Bmse000947
78. Ec 205-702-2
79. (2s)-2-carboxypyrrolidine
80. Schembl7792
81. H-pro-2-chlorotrityl Resin
82. L-proline [usp-rs]
83. (s)-2-pyrralidinecarboxylate
84. (s)-2-pyrrolidinecarboxylate
85. (-)-2-pyrrolidinecarboxylate
86. (s)-2-pyrrolidinecarboxylicaci
87. Proline [ep Monograph]
88. (s)-(-)-prolin
89. Gtpl3314
90. (s)-2-pyrrolidinecarboxylicacid
91. Proline [usp Monograph]
92. Dtxsid5044021
93. L-proline, 99%, Fcc, Fg
94. (s)-2-pyrralidinecarboxylic Acid
95. Pyrrolidin-2-(s)-carboxylic Acid
96. Pharmakon1600-01301007
97. Zinc895360
98. (2s)-pyrrolidin-2-carbonsalphaure
99. Pyrrolidine-2-(s)-carboxylic Acid
100. Bcp25292
101. Hy-y0252
102. (s) -pyrrolidine-2-carboxylic Acid
103. L-proline, >=99.0% (nt)
104. (s)-(-)-pyrrolidine-2-carboxylate
105. Bdbm50000100
106. Ncgc00014017
107. Nsc760114
108. S5629
109. Akos010372120
110. Akos015856025
111. Ccg-214709
112. Cs-w019861
113. Db00172
114. Nsc-760114
115. (s)-(-)-pyrrolidine-2-carboxylic Acid
116. Ncgc00014017-02
117. Ncgc00014017-03
118. Ncgc00097126-01
119. Ac-11190
120. As-10803
121. L-proline, Bioultra, >=99.5% (nt)
122. Db-029981
123. L-proline, Saj Special Grade, >=99.0%
124. Am20080359
125. Bb 0242381
126. L-proline, Vetec(tm), 98.5-101.5%
127. P0481
128. L-proline, Vetec(tm) Reagent Grade, >=99%
129. C00148
130. D00035
131. L-proline, Reagentplus(r), >=99% (hplc)
132. M02947
133. P17692
134. 147p853
135. Q-201327
136. L-proline, Certified Reference Material, Tracecert(r)
137. Q20035886
138. A01b5b63-cc3d-4796-a7b4-c2de26a6fa93
139. F0001-2348
140. Proline, European Pharmacopoeia (ep) Reference Standard
141. Z1245635771
142. L-proline, United States Pharmacopeia (usp) Reference Standard
143. L-proline, Pharmaceutical Secondary Standard; Certified Reference Material
144. L-proline, From Non-animal Source, Meets Ep, Usp Testing Specifications, Suitable For Cell Culture
145. L-proline, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 115.13 g/mol |
|---|---|
| Molecular Formula | C5H9NO2 |
| XLogP3 | -2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 115.063328530 g/mol |
| Monoisotopic Mass | 115.063328530 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 103 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ This study was aimed to evaluate protective and therapeutic effects of a specific mixture, containing vitamin C, lysine, proline, epigallocatechin gallate and zinc, as well as alpha-1-antitrypsin protein on lung tumorigenesis induced by benzo(a) pyrene [B(a)P] in mice. Swiss albino mice were divided into two main experiments, experiment (1) the mice were injected with 100 mg/kg B(a)P and lasted for 28 weeks, while experiment (2) the mice were injected with 8 doses each of 50 mg/kg B(a)P and lasted for 16 weeks. Each experiment (1 and 2) divided into five groups, group (I) received vehicle, group (II) received the protector mixture, group (III) received the carcinogen B(a)P, group (IV) received the protector together with the carcinogen (simultaneously) and group (V) received the carcinogen then the protector (consecutively). Total sialic acid, thiobarbituric acid reactive substances, vascular epithelial growth factor, hydroxyproline levels, as well as elastase and gelatinase activities showed significant elevation in group (III) in the two experiments comparing to control group (P < 0.001). These biochemical alterations were associated with histopathological changes. Administration of the protector in group IV and group V causes significant decrease in such parameters with improvement in histopathological alterations with improvement in histopathological alterations when compared with group III in the two experiments (P < 0.001). The present protector mixture has the ability to suppress neoplastic alteration and restore the biochemical and histopathological parameters towards normal on lung carcinogenesis induced by benzo(a) pyrene in mice. Furthermore, the present mixture have more protective rather than therapeutic action.
PMID:24174951 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3810580 Ibrahim AM et al; J Res Med Sci 18 (5): 427-34 (2013)
L-Proline is extremely important for the proper functioning of joints and tendons and also helps maintain and strengthen heart muscles.
L-Proline is a major amino acid found in cartilage and is important for maintaining youthful skin as well as repair of muscle, connective tissue and skin damage. It is also essential for the immune system, and for necessary balance of this formula. It is an essential component of collagen and is important for proper functioning of joints and tendons. L-Proline is extremely important for the proper functioning of joints and tendons. Helps maintain and strengthen heart muscles.
L-proline is absorbed from the gastrointestinal tract. Ingested dietary protein is denatured in the stomach due to low pH. Denaturing and unfolding of the protein makes the chain susceptible to proteolysis. Up to 15% of dietary protein may be cleaved to peptides and amino acids by pepsins in the stomach. In the duodenum and small intestine digestion continues through hydrolytic enzymes (e.g. trypsin, chymotrypsins, elastase, carboxypeptidase). The resultant mixture of peptides and amino acids is then transported into the mucosal cells by specific carrier systems for amino acids and for di- and tripeptides. The products of digestion are rapidly absorbed. Like other amino acids L-proline is absorbed from ileum and distal jejeunum.
European Chemicals Agency (ECHA); Registered Substances, L-proline (CAS Number: 147-85-3) (EC Number: 205-702-2) (Last updated: December 29, 2015). Available from, as of May 25, 2016: https://echa.europa.eu/
Absorbed peptides are further hydrolyzed resulting in free amino acids which are secreted into the portal blood by specific carrier systems in the mucosal cell. Alternatively they are metabolized within the cell itself. Absorbed amino acids pass into the liver where a portion of the amino acids are used. The remainder pass through into the systemic circulation and are utilized by the peripheral tissue. L-proline is actively transported across the intestine from mucosa to serosal surface. The mechanism of absorption is that of the ion gradient. All L-amino acids are absorbed by Na+dependant, carrier mediated process. This transport is energy dependant by ATP. Plasma L-proline concentrations in normal subjects are reported to be ca. 168 uM/L +/- 60 mM/L with plasma samples collected from healthy volunteers after an overnight fast. As with most nutrients, plasma concentration of L-proline is subject to homeostasis. A number of hormones (e.g., thyroid hormone, catecholamines, and growth hormone) may affect plasma AA levels in diseases. However, in the physiologic state, their influence is probably marginal. However, there is the counter-regulatory hormone system with cortisol and glucagon which influences the blood level of amino acids involved in gluconeogenesis, such as L-proline.
European Chemicals Agency (ECHA); Registered Substances, L-proline (CAS Number: 147-85-3) (EC Number: 205-702-2) (Last updated: December 29, 2015). Available from, as of May 25, 2016: https://echa.europa.eu/
Body losses of amino acids are minimal because amino acids filtered by the kidneys are actively reabsorbed. Also cutaneous losses are negligible. Since there is no long term storage for amino acids in mammals, excess amino acids are degraded, mainly in the liver. Metabolism of amino acids involves removal of the amino group which is converted to urea and excreted in the urine. After removal of the amino group the rest of the acid is utilized as energy source or in anabolism of other endogenous substances. /Amino acids/
European Chemicals Agency (ECHA); Registered Substances, L-proline (CAS Number: 147-85-3) (EC Number: 205-702-2) (Last updated: December 29, 2015). Available from, as of May 25, 2016: https://echa.europa.eu/
Hepatic
L-proline exhibits the same metabolic pathway as several other amino acids do. Metabolism of L-proline is thus described by the entire pathway. This pathway (also known as "Ornithine and Proline Metabolism") describes the co-metabolism of arginine, ornithine, proline, citrulline and glutamate in humans. Arginine is synthesized from citrulline by the sequential action of the cytosolic enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). Citrulline can be derived from ornithine via the catabolism of proline or glutamine/glutamate. Many of the reactions required to generate proline and glutamate from ornithine are located in the mitochondria. Proline is biosynthetically derived from glutamate and its immediate precursor, 1-pyrroline-5-carboxylate. The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage. On a whole-body basis, synthesis of arginine occurs principally via the intestinal-renal axis, wherein epithelial cells of the small intestine, which produce citrulline primarily from glutamine and glutamate, collaborate with the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which is returned to the circulation. Consequently, impairment of small bowel or renal function can reduce endogenous arginine synthesis, thereby increasing the dietary requirement. Both proline and arginine are proteinogenic amino acids and are incorporated into proteins by prolyl-tRNA and arginyl-tRNA, which are synthesized by their respective tRNA synthetases. Arginine can also serve as a precursor for the synthesis of creatine and phopshocreatine through the intermediate guanidoacetic acid. A key component of the arginine/proline metabolic pathway is ornithine. In epithelial cells of the small intestine, ornithine is used primarily to synthesize citrulline and arginine, in liver cells surrounding the portal vein, ornithine functions primarily as an intermediate of the urea cycle, in liver cells surrounding the central vein, ornithine is used to synthesize glutamate and glutamine while in many peripheral tissues, ornithine is used for the synthesis of glutamate and proline.
European Chemicals Agency (ECHA); Registered Substances, L-proline (CAS Number: 147-85-3) (EC Number: 205-702-2) (Last updated: December 29, 2015). Available from, as of May 25, 2016: https://echa.europa.eu/
Glycogenic, by L-Proline oxidase in the kidney, it is ring-opened and is oxidized to form L-Glutamic acid. L-Ornithine and L-Glutamic acid are converted to L-Proline via L-Glutamic acid-gamma-semialdehyde. It is contained abundantly in collagen, and is intimately involved in the function of arthrosis and chordae.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
96
PharmaCompass offers a list of Proline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Proline manufacturer or Proline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Proline manufacturer or Proline supplier.
PharmaCompass also assists you with knowing the Proline API Price utilized in the formulation of products. Proline API Price is not always fixed or binding as the Proline Price is obtained through a variety of data sources. The Proline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Proline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Proline, including repackagers and relabelers. The FDA regulates Proline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Proline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Proline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Proline supplier is an individual or a company that provides Proline active pharmaceutical ingredient (API) or Proline finished formulations upon request. The Proline suppliers may include Proline API manufacturers, exporters, distributors and traders.
click here to find a list of Proline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Proline DMF (Drug Master File) is a document detailing the whole manufacturing process of Proline active pharmaceutical ingredient (API) in detail. Different forms of Proline DMFs exist exist since differing nations have different regulations, such as Proline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Proline DMF submitted to regulatory agencies in the US is known as a USDMF. Proline USDMF includes data on Proline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Proline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Proline suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Proline Drug Master File in Japan (Proline JDMF) empowers Proline API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Proline JDMF during the approval evaluation for pharmaceutical products. At the time of Proline JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Proline suppliers with JDMF on PharmaCompass.
A Proline CEP of the European Pharmacopoeia monograph is often referred to as a Proline Certificate of Suitability (COS). The purpose of a Proline CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Proline EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Proline to their clients by showing that a Proline CEP has been issued for it. The manufacturer submits a Proline CEP (COS) as part of the market authorization procedure, and it takes on the role of a Proline CEP holder for the record. Additionally, the data presented in the Proline CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Proline DMF.
A Proline CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Proline CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Proline suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Proline as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Proline API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Proline as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Proline and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Proline NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Proline suppliers with NDC on PharmaCompass.
Proline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Proline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Proline GMP manufacturer or Proline GMP API supplier for your needs.
A Proline CoA (Certificate of Analysis) is a formal document that attests to Proline's compliance with Proline specifications and serves as a tool for batch-level quality control.
Proline CoA mostly includes findings from lab analyses of a specific batch. For each Proline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Proline may be tested according to a variety of international standards, such as European Pharmacopoeia (Proline EP), Proline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Proline USP).

