



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports

1. H 168 68
2. H 168-68
3. H 16868
4. Magnesium, Omeprazole
5. Omeprazole Magnesium
6. Omeprazole Sodium
7. Prilosec
8. Sodium, Omeprazole
1. 73590-58-6
2. Losec
3. Prilosec
4. Esomeprazole
5. Antra
6. Omeprazon
7. Audazol
8. Omapren
9. Omepral
10. Parizac
11. Zegerid
12. Mopral
13. Miol
14. Belmazol
15. Ceprandal
16. Dizprazol
17. Dudencer
18. Emeproton
19. Epirazole
20. Gastrimut
21. Gastroloc
22. Gibancer
23. Indurgan
24. Inhibitron
25. Inhipump
26. Logastric
27. Pepticum
28. Peptilcer
29. Prazidec
30. Sanamidol
31. Secrepina
32. Ulcometion
33. Mepral
34. Miracid
35. Omeprol
36. Omezol
37. Omisec
38. Omizac
39. Ompanyt
40. Ozoken
41. Prysma
42. Ramezol
43. Ulceral
44. Ulcesep
45. Ulcozol
46. Zefxon
47. Zoltum
48. Desec
49. Elgam
50. Lomac
51. Ulsen
52. Ultop
53. Zimor
54. Ocid
55. Omed
56. Omid
57. Omep
58. Demeprazol
59. Nopramin
60. Omeprazol
61. Omezolan
62. Paprazol
63. Pepticus
64. Prazentol
65. Prazolit
66. Procelac
67. Regulacid
68. Danlox
69. Erbolin
70. Lensor
71. Morecon
72. Nilsec
73. Olexin
74. Omegast
75. Omesek
76. Ortanol
77. Osiren
78. Proclor
79. Result
80. Ulcsep
81. Victrix
82. Zepral
83. Exter
84. Gasec
85. Ulzol
86. Omebeta 20
87. Tedec Ulceral
88. Aulcer
89. Antra Mups
90. Omerprazole
91. Omez
92. 119141-88-7
93. Omepradex
94. Omeprazolum
95. H 168/68
96. Gastrogard
97. Nuclosina
98. Omesec
99. Omeprazole-d3
100. Mfcd00083192
101. Prestwick_808
102. 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole
103. 6-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1h-benzo[d]imidazole
104. H-168/68
105. Omz
106. Chebi:77260
107. 119141-89-8
108. 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1h-benzimidazole
109. 1h-benzimidazole, 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-
110. Aisi'aomeilazuona Esomeprazole Sodium
111. Nsc-751450
112. Nsc-759192
113. Chembl1503
114. Nexiam
115. Esomeprazole Sodium Salt
116. 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-1,3-benzodiazole
117. 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1h-benzimidazole
118. 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1h-benzimidazole
119. Mls000069373
120. Kg60484qx9
121. 73590-58-6 (free Form)
122. Omeprazol [inn-spanish]
123. Omeprazolum [inn-latin]
124. Ncgc00016925-06
125. Esomperazole
126. Smr000058847
127. Emilok
128. Cas-73590-58-6
129. Dsstox_cid_1080
130. 2-({[3,5-dimethyl-4-(methyloxy)pyridin-2-yl]methyl}sulfinyl)-5-(methyloxy)-1h-benzimidazole
131. 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methane]sulfinyl}-1h-1,3-benzodiazole
132. Dsstox_rid_75929
133. Dsstox_gsid_21080
134. ( -)-omeprazole
135. (r)-5-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1h-benzo[d]imidazole
136. Omeprazone
137. Omeprazen
138. Omeprazole Delayed-release
139. Omeprazole Pellets
140. 5-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1h-benzo[d]imidazole
141. 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1h-benzo[d]imidazole
142. 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1h-benzimidazole
143. 5-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-3h-benzoimidazole
144. 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]benzimidazole
145. Prilosec (tn)
146. (+-)-omeprazole
147. Ccris 7099
148. Hsdb 3575
149. Sr-01000003003
150. Unii-kg60484qx9
151. Omeprazole-d6
152. Omeprazole, Solid
153. 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1h-benzimidazole
154. Agi-010
155. Omeprazole,(s)
156. Omeprazole Solution
157. Omeprazole-[d3]
158. (rs)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) Methylsulfinyl)-1h-benzo(d)imidazole
159. (rs)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) Methylsulfinyl)-1h-benzo[d]imidazole
160. 326602-80-6
161. Omeprazole S-isomer
162. 2-(((3,5-dimethyl-4-methoxy-2-pyridyl)methyl)sulfinyl)-5-methoxy-1h-benzimidazole
163. Omeprazole - Bio-x
164. Dm-3458
165. Omeprazole [usan:usp:inn:ban:jan]
166. Omeprazole-13c,d3
167. Omeprazole (prilosec)
168. Omeprazole [mi]
169. Omeprazole [inn]
170. Omeprazole [jan]
171. Maybridge4_002645
172. Opera_id_1863
173. Prestwick0_000493
174. Prestwick1_000493
175. Prestwick2_000493
176. Prestwick3_000493
177. (.+/-.)-omeprazole
178. Omeprazole [hsdb]
179. Omeprazole [usan]
180. O0359
181. Omeprazole [vandf]
182. Upcmld-dp075
183. Cid_4594
184. Omeprazole [mart.]
185. Schembl1191
186. Omeprazole [usp-rs]
187. Omeprazole [who-dd]
188. H 16868
189. Bspbio_000385
190. Mls001076112
191. Mls001424148
192. Mls006010400
193. Mls006011759
194. Bidd:gt0189
195. Spbio_002306
196. Bpbio1_000425
197. Gtpl4279
198. Omeprazole (jp17/usp/inn)
199. Omeprazole [green Book]
200. Dtxsid6021080
201. Omeprazole [orange Book]
202. Schembl11995456
203. Upcmld-dp075:001
204. Chebi:91766
205. Omeprazole [usp Impurity]
206. Hms1528i05
207. Hms1569d07
208. Hms2052g17
209. Hms2090e16
210. Hms2090f11
211. Hms2096d07
212. Hms2232b21
213. Hms3269d17
214. Hms3394g17
215. Hms3413j07
216. Hms3651a11
217. Hms3677j07
218. Hms3713d07
219. Omeprazole [usp Monograph]
220. Pharmakon1600-01505693
221. Zegerid Component Omeprazole
222. Amy30573
223. Bcp05852
224. Bcp13592
225. Bcp21299
226. Hy-b0113
227. Yosprala Component Omeprazole
228. 2,3,5-trimethylpyridine/omeprazole
229. Tox21_110686
230. Tox21_200509
231. Ac-401
232. Bbl028172
233. Bdbm50103597
234. Bdbm50241343
235. Dl-462
236. Mfcd23135254
237. Nsc751450
238. Nsc759192
239. S1389
240. Stk623746
241. Akos005066653
242. Akos015895343
243. Omeprazole Component Of Zegerid
244. Tox21_110686_1
245. Ac-4676
246. Ccg-101130
247. Ccg-213517
248. Cs-1868
249. Db00338
250. Hs-0055
251. Nc00380
252. Nsc 751450
253. Nsc 759192
254. Omeprazole Component Of Yosprala
255. (s)-5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-3h-benzoimidazole
256. Idi1_032523
257. Ncgc00016925-01
258. Ncgc00016925-02
259. Ncgc00016925-03
260. Ncgc00016925-04
261. Ncgc00016925-05
262. Ncgc00016925-07
263. Ncgc00016925-08
264. Ncgc00016925-10
265. Ncgc00016925-11
266. Ncgc00021522-03
267. Ncgc00021522-04
268. Ncgc00021522-05
269. Ncgc00258063-01
270. Omeprazole, Analytical Reference Material
271. Bo164173
272. Sy009746
273. Sy077145
274. Sbi-0206896.p001
275. Ft-0601585
276. Ft-0652860
277. Ft-0653294
278. Ft-0673283
279. Ft-0689771
280. H 199
281. Sw196942-4
282. A19447
283. C07324
284. D00455
285. 141o887
286. A837865
287. A892647
288. A937349
289. Q422210
290. Sr-01000003003-4
291. Sr-01000003003-7
292. Sr-01000003003-8
293. 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)
294. Brd-a55962179-001-04-9
295. Brd-a55962179-001-08-0
296. Brd-a55962179-001-20-5
297. Brd-a88691025-001-07-4
298. F0001-2386
299. Z1672902589
300. Omeprazole, British Pharmacopoeia (bp) Reference Standard
301. Omeprazole, European Pharmacopoeia (ep) Reference Standard
302. Omeprazole, United States Pharmacopeia (usp) Reference Standard
303. 2-(3-methoxy-2,4-dimethylbenzylsulfinyl)-6-methoxy-1h-benzo[d]imidazole
304. 5-methoxy-2-((s)-((4-methoxy-3,5-dimethyl-2- Pyridinyl)methyl)sulfinyl)-
305. Omeprazole, Pharmaceutical Secondary Standard; Certified Reference Material
306. (+)-5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-sulfinyl]-1h-benzimidazole
307. (-)-5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-sulfinyl]-1h-benzimidazole
308. (rs)-5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridylmethylsulphinyl)benzimidazole
309. 1h-benzimidazole,5-methoxy-2-[(r)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-
310. 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyrdinyl)-methyl]sulfinyl]-1h-benzimidazole
311. 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1 H-benzimidazole
312. 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole
313. 5-methoxy-2-(2-(4-methoxy-3,5-dimethylpyridin-2-yl)ethylsulfinyl)-1h-benzo[d]imidazole
314. 5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1h-benzoimidazole
315. 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl- 1h-benzimidazole
316. 5-methoxy-2-[[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl]sulfinyl]-1h-benzimidazole
317. 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulphinyl]-1h-benzimidazole
318. 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-benzimidazole
319. 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulphinyl]1h-benzimidazole
320. 6-methoxy-2-(((4-methoxy-3,5-dimethyl Pyridin-2-yl)methyl)sulfinyl)-1h-benzo[d]imidazole
321. 6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1h-benzo[d]imidazole
322. 6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1h-benzimidazole
323. 6-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-1h-benzimidazole
324. 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole
325. 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1h-benzimidazole
326. 6-methoxy-2-[[(4-methoxy-3,5dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole
327. 6-methoxy-2-[[(4-methoxy3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole
328. 6-methoxy-2-[[(4methoxy-3,5-dimethyl2-pyridinyl)methyl]sulfinyl]-1h-benzimidazole
329. Omeprazole For Peak Identification, European Pharmacopoeia (ep) Reference Standard
330. Omeprazole Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
331. (omeprazole)5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1h-benzoimidazole
332. 1h-benzimidazole,6-methoxy-2-[(r)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-
333. 5-methoxy-2-(((4-methoxy-3,5,-dimethyl-2-pyridinyl)-methyl)sulphinyl)-1h-benzimidazole
334. 5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1h-benzoimidazole (omeprazole)
335. 5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1h-benzoimidazole(omeprazole)
| Molecular Weight | 345.4 g/mol |
|---|---|
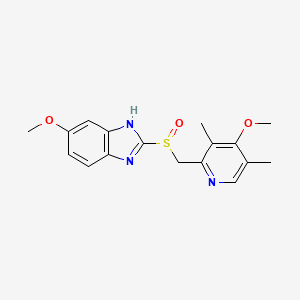
| Molecular Formula | C17H19N3O3S |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 345.11471265 g/mol |
| Monoisotopic Mass | 345.11471265 g/mol |
| Topological Polar Surface Area | 96.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 14 | |
|---|---|
| Drug Name | Nexium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 2 of 14 | |
|---|---|
| Drug Name | Nexium iv |
| PubMed Health | Omeprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in Omeprazole Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formul... |
| Active Ingredient | Esomeprazole sodium |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 20mg base/vial; eq 40mg base/vial |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 14 | |
|---|---|
| Drug Name | Omeprazole |
| PubMed Health | Omeprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | 11 DESCRIPTIONThe active ingredient in PRILOSEC (omeprazole) Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secret... |
| Active Ingredient | Omeprazole |
| Dosage Form | Capsule, delayed rel pellets; Tablet, delayed release |
| Route | oral; Oral |
| Strength | 10mg; 40mg; 20mg |
| Market Status | Tentative Approval; Over the Counter; Prescription |
| Company | Actavis Labs Fl; Apotex; Sandoz; Kremers Urban Pharms; Zydus Pharms Usa; Dr Reddys Labs; Dexcel Pharma; Mylan; Impax Labs |
| 4 of 14 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| PubMed Health | Omeprazole/Sodium Bicarbonate (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | 11 DESCRIPTIONThe active ingredient in PRILOSEC (omeprazole) Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secret... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
| 5 of 14 | |
|---|---|
| Drug Name | Prilosec |
| Active Ingredient | Omeprazole magnesium; Omeprazole |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 10mg base/packet; 10mg; 40mg; 20mg; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 6 of 14 | |
|---|---|
| Drug Name | Prilosec otc |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Astrazeneca |
| 7 of 14 | |
|---|---|
| Drug Name | Zegerid |
| Active Ingredient | sodium bicarbonate; Omeprazole |
| Dosage Form | Capsule; For suspension |
| Route | Oral |
| Strength | 20mg/packet; 1.1gm; 40mg/packet; 1.68gm/packet; 40mg; 20mg |
| Market Status | Prescription |
| Company | Santarus |
| 8 of 14 | |
|---|---|
| Drug Name | Nexium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 9 of 14 | |
|---|---|
| Drug Name | Nexium iv |
| PubMed Health | Omeprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in Omeprazole Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formul... |
| Active Ingredient | Esomeprazole sodium |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 20mg base/vial; eq 40mg base/vial |
| Market Status | Prescription |
| Company | Astrazeneca |
| 10 of 14 | |
|---|---|
| Drug Name | Omeprazole |
| PubMed Health | Omeprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | 11 DESCRIPTIONThe active ingredient in PRILOSEC (omeprazole) Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secret... |
| Active Ingredient | Omeprazole |
| Dosage Form | Capsule, delayed rel pellets; Tablet, delayed release |
| Route | oral; Oral |
| Strength | 10mg; 40mg; 20mg |
| Market Status | Tentative Approval; Over the Counter; Prescription |
| Company | Actavis Labs Fl; Apotex; Sandoz; Kremers Urban Pharms; Zydus Pharms Usa; Dr Reddys Labs; Dexcel Pharma; Mylan; Impax Labs |
| 11 of 14 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| PubMed Health | Omeprazole/Sodium Bicarbonate (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | 11 DESCRIPTIONThe active ingredient in PRILOSEC (omeprazole) Delayed-Release Capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secret... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
| 12 of 14 | |
|---|---|
| Drug Name | Prilosec |
| Active Ingredient | Omeprazole magnesium; Omeprazole |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 10mg base/packet; 10mg; 40mg; 20mg; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 13 of 14 | |
|---|---|
| Drug Name | Prilosec otc |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Astrazeneca |
| 14 of 14 | |
|---|---|
| Drug Name | Zegerid |
| Active Ingredient | sodium bicarbonate; Omeprazole |
| Dosage Form | Capsule; For suspension |
| Route | Oral |
| Strength | 20mg/packet; 1.1gm; 40mg/packet; 1.68gm/packet; 40mg; 20mg |
| Market Status | Prescription |
| Company | Santarus |
Anti-Ulcer Agents; Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Omeprazole is indicated for the treatment of a complex of symptoms which may be caused by any of the conditions where a reduction of gastric acid secretion is required (e.g., duodenal ulcer, gastric ulcer, nonsteroidal anti-inflammatory drugs associated gastric and duodenal ulcer, reflux esophagitis, gastroesophageal reflex disease) or when no identifiable organic cause is found (i.e., functional dyspepsia). /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2131
Omeprazole is indicated for the treatment of heartburn and other symptoms associated with gastroesophageal reflux disease. Omeprazole is indicated for the short-term treatment of erosive esophagitis (associated with gastroesophageal reflux disease) that has been diagnosed by endoscopy. Omeprazole also is indicated to maintain healing of erosive esophagitis. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2131
Omeprazole is indicated for the long-term treatment of pathologic gastric hypersecretion associated with Zollinger-Ellison syndrome (alone or as part of multiple endocrine neoplasia Type-1), systemic mastocytosis, and multiple endocrine adenoma. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2131
For more Therapeutic Uses (Complete) data for OMEPRAZOLE (8 total), please visit the HSDB record page.
Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
No information is available on the relationship of age to the effects of omeprazole in geriatric patients. However, a somewhat decreased rate of elimination and increased bioavailability are more likely to occur in geriatric patients taking omeprazole.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
Omeprazole generally is well tolerated. The most frequent adverse effects associated with omeprazole therapy involve the GI tract (e.g., diarrhea, nausea, constipation, abdominal pain, vomiting) and the CNS (e.g., headache, dizziness).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2839
Diarrhea, abdominal pain, nausea, vomiting, constipation, flatulence, and acid regurgitation are the most frequent adverse GI effects of omeprazole, occurring in about 1-5% of patients. Dysphagia, abdominal swelling, anorexia, irritable colon, fecal discoloration, pancreatitis (sometimes fatal), esophageal candidiasis, mucosal atrophy of the tongue, taste perversion, and dry mouth have been reported occasionally but in many cases were not directly attributed to the drug. Benign gastric fundic polyps have been reported rarely and appear to resolve upon discontinuation of omeprazole therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2840
For more Drug Warnings (Complete) data for OMEPRAZOLE (15 total), please visit the HSDB record page.
Omeprazole, according to the FDA label is a proton pump inhibitor (PPI) used for the following purposes: Treatment of active duodenal ulcer in adults Eradication of Helicobacter pylori to reduce the risk of duodenal ulcer recurrence in adults Treatment of active benign gastric ulcer in adults Treatment of symptomatic gastroesophageal reflux disease (GERD) in patients 1 year of age and older Treatment of erosive esophagitis (EE) due to acid-mediated GERD in patients 1 month of age and older Maintenance of healing of EE due to acid-mediated GERD in patients 1 year of age and older Pathologic hypersecretory conditions in adults
FDA Label
**Effects on gastric acid secretion** This drug decreases gastric acid secretion. After oral administration, the onset of the antisecretory effect of omeprazole is usually achieved within one hour, with the maximum effect occurring by 2 hours after administration. The inhibitory effect of omeprazole on acid secretion increases with repeated once-daily dosing, reaching a plateau after four days. **Effects on serum gastrin** In studies of 200 or more patients, serum gastrin levels increased during the first 1-2 weeks of daily administration of therapeutic doses of omeprazole. This occurred in a parallel fashion with the inhibition of acid secretion. No further increase in serum gastrin occurred with continued omeprazole administration. Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum Chromogranin A (CgA) levels. The increased CgA levels may lead to false positive results in diagnostic studies for neuroendocrine tumors. **Enterochromaffin-like (ECL) cell effects** Human gastric biopsy samples have been obtained from more than 3000 pediatric and adult patients treated with omeprazole in long-term clinical studies. The incidence of enterochromaffin-like cell hyperplasia in these studies increased with time; however, no case of ECL cell carcinoids, dysplasia, or neoplasia have been identified in these patients. These studies, however, are of insufficient in power and duration to draw conclusions on the possible influence of long-term administration of omeprazole in the development of any premalignant or malignant conditions. **Other effects** Systemic effects of omeprazole in the central nervous system, cardiovascular and respiratory systems have not been found to date. Omeprazole, given in oral doses of 30 or 40 mg for 2-4 weeks, showed no effect on thyroid function, carbohydrate metabolism, or circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin or secretin.
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)
A02BC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BC - Proton pump inhibitors
A02BC01 - Omeprazole
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BC - Proton pump inhibitors
A02BC05 - Esomeprazole
Absorption
Omeprazole delayed-release capsules contain an enteric-coated granule formulation of omeprazole (because omeprazole is acid-labile), so that absorption of omeprazole begins only after the granules exit the stomach. Absorption of omeprazole occurs rapidly, with peak plasma concentrations of omeprazole achieved within 0.5-3.5 hours. Absolute bioavailability (compared with intravenous administration) is approximately 30-40% at doses of 20-40 mg, largely due to pre-systemic metabolism. The bioavailability of omeprazole increases slightly upon repeated administration of omeprazole delayed-release capsules.
Route of Elimination
After a single dose oral dose of a buffered solution of omeprazole, negligible (if any) amounts of unchanged drug were excreted in urine. Most of the dose (about 77%) was eliminated in urine as at least six different metabolites. Two metabolites were identified as _hydroxyomeprazole_ and the corresponding _carboxylic acid_. The remainder of the dose was found in the feces. This suggests significant biliary excretion of omeprazole metabolites. Three metabolites have been identified in the plasma, the _sulfide_ and _sulfone_ derivatives of omeprazole, and _hydroxyomeprazole_. These metabolites possess minimal or no antisecretory activity.
Volume of Distribution
Approximately 0.3 L/kg, corresponding to the volume of extracellular water.
Clearance
Healthy subject (delayed release capsule), total body clearance 500 - 600 mL/min Geriatric plasma clearance: 250 mL/min Hepatic impairment plasma clearance: 70 mL/min
Absorption: rapid. Distribution: Distributed in tissue, particularly gastric parietal cells.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
Elimination: Renal 72 to 80%. Fecal 18 to 23%. In dialysis - Not readily dialyzable, because of extensive protein binding.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
To clarify the in vivo first-pass metabolism of omeprazole, the pharmacokinetics were examined after oral, intraduodenal, intraportal venous, and intravenous administration at various doses to rats. Extraction ratios in the liver and intestinal tract were determined from the areas under the concentration-time curve (AUC) for intraportal venous and intravenous administration and from those for intraduodenal and intraportal venous administration, respectively. Assuming that the drug was absorbed from the gastrointestinal tract completely, the hepatic and intestinal extraction ratios were 0.80, 0.63, and 0.59 at doses of 2.5, 5, and 10 mg/kg and 0.70 and 0.73 at doses of 5 and 10 mg/kg, respectively. The bioavailability of orally administered omeprazole was 6.4, 9.6, and 12.6% at the doses of l0, 20, and 40 mg/kg, respectively. There were no differences in the distribution volume of steady state, total clearance, or elimination half-life at any doses. In addition, the AUC value after oral administration (20 mg/kg) in rats acutely intoxicated with CC(14) was 2.4 times larger than that in the control. These findings suggest that omeprazole undergoes a first-pass metabolism in the intestinal mucosa and/or lumen, as well as in the liver, and that the major contribution to the dose-dependent increase in bioavailability is a saturation of the first-pass metabolism in the liver.
PMID:7983597 Watanabe K et al; J Pharm Sci 83 (8): 1131-4 (1994)
Omeprazole is distributed into human milk; following oral administration of omeprazole 20 mg in one lactating women, concentrations of the drug were measured in breast milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2841
For more Absorption, Distribution and Excretion (Complete) data for OMEPRAZOLE (6 total), please visit the HSDB record page.
Omeprazole is heavily metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The main part of its metabolism depends on the polymorphically expressed CYP2C19, which is responsible for the formation of _hydroxyomeprazole_, the major metabolite found in plasma. The remaining part depends on CYP3A4, responsible for the formation of _omeprazole sulphone_.
The in vitro metabolism of omeprazole was studied in human liver microsomes in order to define the metabolic pathways and identify the cytochrome P450 (CYP) isoforms responsible for the formation of the major omeprazole metabolites. 2 The four major metabolites identified in vitro, in tentative order of importance, were hydroxyomeprazole, omeprazole sulphone, 5-O-desmethylomeprazole, and an unidentified compound termed metabolite X. Omeprazole pyridone was also detected but could not be quantitated. Incubation of hydroxyomeprazole and omeprazole sulphone with human microsomes resulted in both cases in formation of the hydroxysulphone. The kinetics of formation of the four primary metabolites studied were biphasic suggesting the involvement of multiple CYP isoforms in each case. Further studies used substrate concentrations at which the high affinity activities predominated. 3 Formation of the major metabolite, hydroxyomeprazole, was significantly correlated with S-mephenytoin hydroxylase and with benzo[a]pyrene metabolism and CYP3A content. Inhibition studies with isoform selective inhibitors also indicated a dominant role of S-mephenytoin hydroxylase with some CYP3A contribution in the formation of hydroxyomeprazole. Correlation and inhibition data for the sulphone and metabolite X were consistent with a predominant role of the CYP3A subfamily in formation of these metabolites. Formation of 5-O-desmethylomeprazole was inhibited by both R, S-mephenytoin and quinidine, indicating that both S-mephenytoin hydroxylase and CYP2D6 may mediate this reaction in human liver microsomes and in vivo. The Vmax/Km (indicator of intrinsic clearance in vivo) for hydroxyomeprazole was four times greater than that for omeprazole sulphone. Consistent with findings in vivo, the results predict that omeprazole clearance in vivo would be reduced in poor metabolisers of mephenytoin due to reduction in the dominant partial metabolic clearance to hydroxyomeprazole.
PMID:12959268 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1364656 Andersson T et al; Br J Clin Pharmacol 36 (6): 521-30 (1993)
Omeprazole has known human metabolites that include 3-Hydroxyomeprazole, 5'-O-Desmethyl omeprazole, 5-Hydroxyomeprazole, and Omeprazole sulfone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
0.5-1 hour (healthy subjects, delayed-release capsule) Approximately 3 hours (hepatic impairment)
Plasma - Normal hepatic function - 30 minutes to 1 hour. Chronic hepatic disease - 3 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
Hydrochloric acid (HCl) secretion into the gastric lumen is a process regulated mainly by the H(+)/K(+)-ATPase of the proton pump, expressed in high quantities by the parietal cells of the stomach. ATPase is an enzyme on the parietal cell membrane that facilitates hydrogen and potassium exchange through the cell, which normally results in the extrusion of potassium and formation of HCl (gastric acid). Omeprazole is a member of a class of antisecretory compounds, the substituted _benzimidazoles_, that stop gastric acid secretion by selective inhibition of the _H+/K+ ATPase_ enzyme system. Proton-pump inhibitors such as omeprazole bind covalently to cysteine residues via disulfide bridges on the alpha subunit of the _H+/K+ ATPase_ pump, inhibiting gastric acid secretion for up to 36 hours. This antisecretory effect is dose-related and leads to the inhibition of both basal and stimulated acid secretion, regardless of the stimulus. **Mechanism of H. pylori eradication** Peptic ulcer disease (PUD) is frequently associated with _Helicobacter pylori_ bacterial infection (NSAIDs). The treatment of H. pylori infection may include the addition of omeprazole or other proton pump inhibitors as part of the treatment regimen,. _H. pylori_ replicates most effectively at a neutral pH. Acid inhibition in H. pylori eradication therapy, including proton-pump inhibitors such as omeprazole, raises gastric pH, discouraging the growth of H.pylori. It is generally believed that proton pump inhibitors inhibit the _urease_ enzyme, which increases the pathogenesis of H. pylori in gastric-acid related conditions.
Omeprazole is a selective and irreversible proton pump inhibitor. Omeprazole suppresses gastric acid secretion by specific inhibition of the hydrogen-potassium adenosinetriphosphatase (H+, K+-ATPase) enzyme system found at the secretory surface of parietal cells. It inhibits the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen. Since the H+/K+ ATPase enzyme system is regarded as the acid (proton) pump of the gastric mucosa, omeprazole is known as a gastric acid pump inhibitor. Omeprazole inhibits both basal and stimulated acid secretion irrespective of the stimulus.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2132
After oral administration, the onset of the antisecretory effect of omeprazole occurs within one hour, with the maximum effect occurring within two hours. Inhibition of secretion is about 50% of maximum at 24 hours and the duration of inhibition lasts up to 72 hours. The antisecretory effect thus lasts far longer than would be expected from the very short (less than one hour) plasma half-life, apparently due to prolonged binding to the parietal H + /K + ATPase enzyme. When the drug is discontinued, secretory activity returns gradually, over 3 to 5 days. The inhibitory effect of omeprazole on acid secretion increases with repeated once-daily dosing, reaching a plateau after four days.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 634








