


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
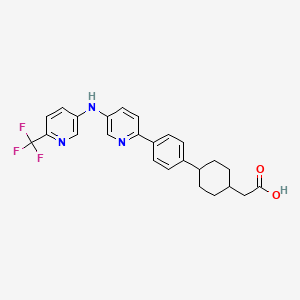

1. Lcq908
1. Lcq-908
2. 956136-95-1
3. Lcq908
4. Pradigastat Free Acid
5. Lcq-908nxa
6. Lcq908-nxa
7. Lcq-908-nxa
8. 2u23g6vnuz
9. Lcq908 - Dgat-1-inhibitor
10. 956136-95-1 (free Acid)
11. Pradigastat (usan)
12. Lcq 908
13. Pradigastat [usan]
14. 2-((1s,4s)-4-(4-(5-((6-(trifluoromethyl)pyridin-3-yl)amino)pyridin-2-yl)phenyl)cyclohexyl)acetic Acid
15. 2-[4-[4-[5-[[6-(trifluoromethyl)pyridin-3-yl]amino]pyridin-2-yl]phenyl]cyclohexyl]acetic Acid
16. 2-{4-[4-(5-{[6-(trifluoromethyl)pyridin-3-yl]amino}pyridin-2-yl)phenyl]cyclohexyl}acetic Acid
17. Pradigastat [inn]
18. Pradigastat [usan:inn]
19. Unii-2u23g6vnuz
20. Lcq 908nxa
21. Schembl180536
22. Gtpl7830
23. Schembl1289309
24. Chembl2364624
25. Schembl16104874
26. Schembl18286769
27. Dtxsid401026085
28. Bcp21089
29. Lcq908; Lcq 908; Pradigastat
30. Bdbm50502586
31. Zinc253387875
32. Cs-1222
33. Db12866
34. Sb16971
35. Ncgc00378933-01
36. Ncgc00378933-02
37. Hy-16278
38. Db-125833
39. D10664
40. A900338
41. Q27088438
42. (4-{4-[5-(6-trifluoromethyl-pyridin-3-ylamino)-pyridin-2-yl]-phenyl}-cyclohexyl)-acetic Acid
43. Cyclohexaneacetic Acid, 4-(4-(5-((6-(trifluoromethyl)-3-pyridinyl)amino)-2-pyridinyl)phenyl)-, Trans-
44. Lcq-908; Trans-4-[4-[5-[[6-(trifluoromethyl)-3-pyridinyl]amino]-2-pyridinyl]phenyl]cyclohexaneacetic Acid;lcq-908
45. Trans-4-[4-[5-[[6-(trifluoromethyl)-3-pyridinyl]amino]-2-pyridinyl]phenyl]cyclohexaneacetic Acid
| Molecular Weight | 455.5 g/mol |
|---|---|
| Molecular Formula | C25H24F3N3O2 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 455.18206150 g/mol |
| Monoisotopic Mass | 455.18206150 g/mol |
| Topological Polar Surface Area | 75.1 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 631 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of familial chylomicronaemia syndrome (type I hyperlipoproteinaemia


